Project Description
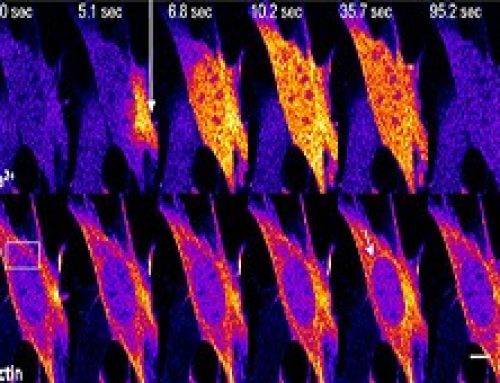
Clathrin-mediated endocytosis (CME) occurs as periodic traveling waves, being co-ordinated spatially and temporally through the nucleation of actin waves. The link between endocytosis and generation of cortical actin waves may provide deeper insight into how cells maintain the composition and dynamics of the plasma membrane
These findings were published in Developmental Cell in 2017.
Yang Y, Ding X, Pipathsouk A, Weiner OD, Wu M. (2017) Clathrin Assembly Defines the Onset and Geometry of Cortical Patterning. 43(4):507-521.e4. doi: 10.1016/j.devcel.2017.10.028
More information on the Wu Lab
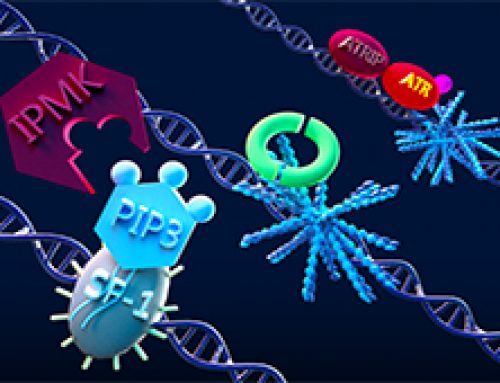
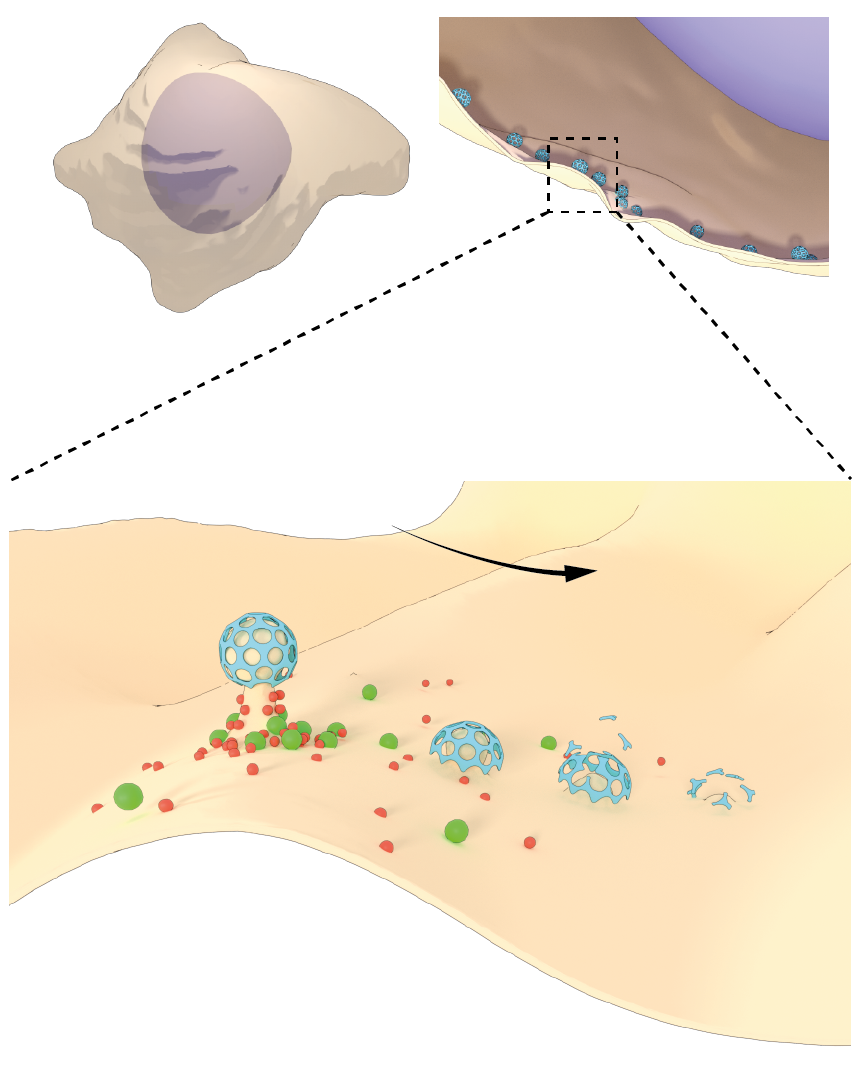
Figure: The top left of the figure depicts a cell with clathrin-mediated endocytic waves. The top right depics a cross section of the cell with clathrin assembly in process through collective dynamics. Onset of clathrin waves require intermediate levels of PIP3 (red) as well as feedback from downstream components of cortical wave machinery such as FBP17 (green). The arrow represents direction of propagation of clathrin waves.
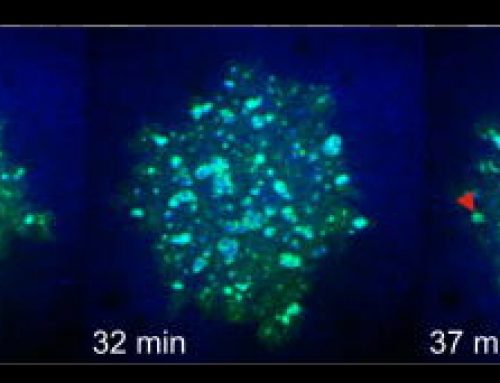
Clathrin- Mediated Endocytosis (CME) was previously established as a random endocytic event with no spatial or temporal coordination of events. However, this study reveals the existence of collective dynamics of CME, where the individual events of CME such as clathrin nucleation, clathrin coated pit (CCP) formation, recruitment of F-BAR proteins, Cdc42 and N-WASP, are synchronized and appear as periodic traveling waves. The observed collective dynamics of CME occurs when coupled through feedback between clathrin and downstream players of CME such as Cdc42/F-BAR. This study also reveals a role for clathrin in organizing the geometry of cortical waves in addition to regulating its onset.
Understanding the basics
Clathrin-mediated endocytosis (CME) is a vesicular transport event that facilitates the internalization and recycling of receptors engaged in a variety of processes, including signal transduction (G-protein and tyrosine kinase receptors), nutrient uptake and synaptic vesicle reformation. Two classical examples of CME are iron-bound transferrin recycling and the uptake of low-density lipoprotein (LDL). CME is characterized by the involvement of clathrin, which is a triskelion-shaped scaffold protein composed of three heavy and three light chains. Clathrins polymerize around the cytoplasmic face of the invaginated membrane and act as a reinforced mould in which the membrane vesicle may form. Initiation of the clathrin complex formation requires the accumulation of phosphatidylinositol‑4,5‑bisphosphate (PIP2) and adaptor proteins, such as AP-2, at the pinching site.
Three main types of phosphoinositides have important roles in intracellular signaling, lipid signaling, and membrane trafficking; these phosphoinositides differ solely in the number of phosphate groups that are attached by phosphoinositol kinases to the inositol ring. 1) Phosphatidylinositol-4-phosphate (PIP) – increased levels of PIP in the plasma membrane greatly reduces the F-actin binding and depolymerizing activity of ADF (actin depolymerizing factor). 2) Phosphatidylinositol-4,5-bis-phosphate (PIP2) – increased levels of PIP2 in the plasma membrane inhibits actin filament capping by capping protein and greatly reduces the F-actin binding and depolymerizing activity of ADF. 3) Phosphatidylinositol-3,4,5-trisphosphate (PIP3) – Phosphatidylinositol-3-kinase (PI3K) and PTEN (Phosphatase and tensin homolog) signal transduction pathways regulate the level of PIP3 in response to extracellular guidance cues during filopodia motility. The accumulation of PIP3 in filopodia is suggested to cause actin polymerization and increased cellular movement.
Cortical actin waves are wave-like movement of actin filaments and associated proteins underlying the cells. Actin waves play important roles in cellular protrusion, polarization and migration.