Numerous motor proteins exist and each possess unique characteristics that allow them to facilitate different processes and functions. Even within the myosin superfamily variation exists in structure and function of each member. Not only can motor proteins translocate along microfilaments, but they can induce movement in the filament itself. It is this property that gives rise to the contractile properties of skeletal muscle.
Contractile Bundles in Skeletal Muscle
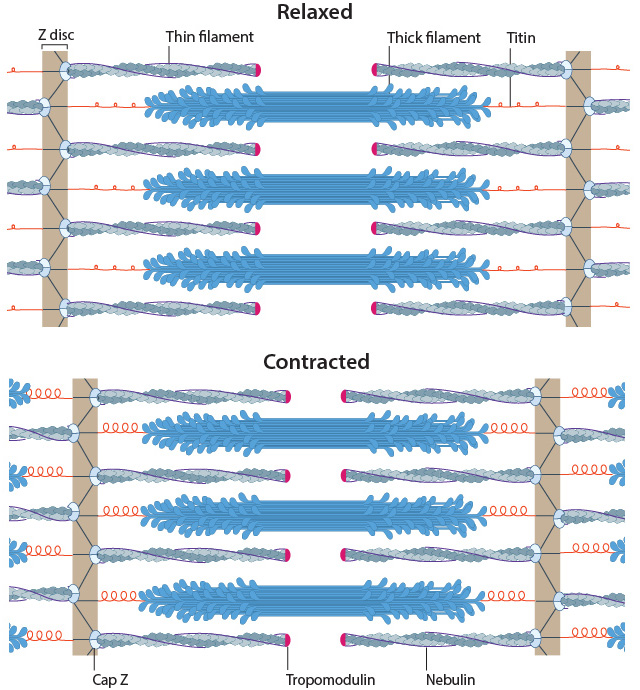
Muscle sarcomeres during relaxation and contraction
In skeletal muscle cells, myosin II forms only thick filaments that are arranged within a scaffold of actin thin filaments (along with numerous other proteins). These form the higher order fibrous structures known as sarcomeres. Each sarcomere contains numerous repeating units of interlinked thick and thin filaments, and the opposite orientation of the myosin heads causes adjacent actin filaments to slide past each other during muscle contraction. Each sarcomere is ~2 µm long in resting muscle, but this length is shortened by as much as 70% after muscle contraction. Muscle contraction is regulated by calcium levels [1] and by the troponin regulatory system. Although actin subunits continue to turn-over at both ends of the thin filament, this exchange is relatively slow, making the actin filaments in sarcomeres relatively more stable when compared to the actin filaments found in other cell types.
Contractile Bundles in Nonmuscle Cells
In non-muscle cells, myosin II associates with actin filaments to form contractile structures known as stress fibers along the lower surfaces where the cell is anchored to its substrate. In epithelial cells, contractile bundles are also prominent in the adhesion belt (aka adherens belt), which helps to maintain the stability and integrity of epithelial cell sheets. The contractile bundles in nonmuscle cells are similar to skeletal muscle fibers, but they are smaller (~0.4 µm in fibroblasts), less organized, and they contain different accessory proteins [2].
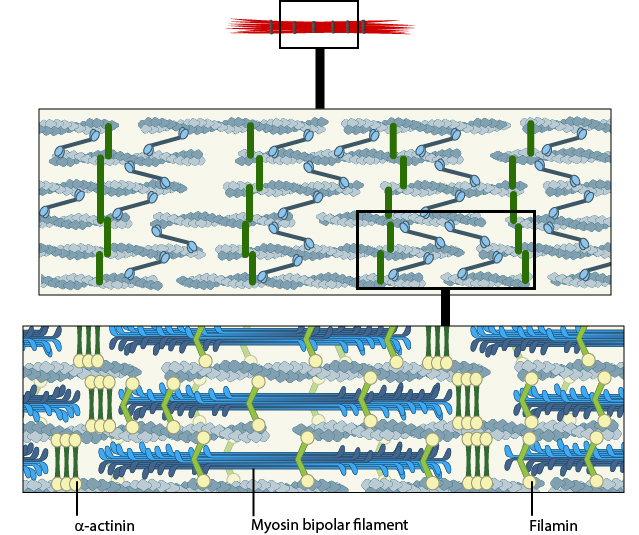
A. Isolated stress fibers have a banded appearance, with bundles of actin filaments interspersed with semiperiodic electron-dense regions. B. The electron-dense regions are rich in actin crosslinking proteins, namely α-actinin. Bipolar myosin II filaments lie between the loosely packed actin filaments in the regions that lack α-actinin . C. A high resolution view of the bipolar myosin filament heads interspersed between the regions rich in α-actinin. Relative to α-actinin, the more flexible actin crosslinking protein, filamin, is dispersed throughout the entire stress fiber.
Historically speaking, the mechanism of actomyosin contraction for nonmuscle actin was examined using amoebae proteins Dictyostelium, Acanthamoeba) because the actin is very similar to muscle actin [3]; these initial studies showed the rate of ATP hydrolysis by myosin (and hence myosin movement) varies directly with the actin concentration [4]. Further studies using isolated stress fibers from fibroblasts confirmed that stress fibers are contractile and shorten by as much as 25% [5]. Myosin II bundle formation and contractile activity in nonmuscle cells is regulated by phosphorylation [6].
Related Questions
Each myosin motor protein possesses ATPase activity and functions in a cyclical manner that couples ATP binding and hydrolysis to a conformational change in the protein. This process is known as the ‘powerstroke cycle’… Read more…
Several myosin isoforms have been found in eukaryotes, each differing in the type of heavy and light chains they are composed of. All myosins are composed of a diverse ‘tail’ domain at their carboxy terminus and an evolutionarily conserved globular ‘head’ domain at their amino terminus… Read more…
The most commonly described motor proteins belong to the Myosin superfamily. Myosin II can form higher order assemblies via the extended coiled-coil domains in the heavy chains and is known for enabling contraction in muscle cells when in complex with actin filaments. In non-muscle cells, actin filaments form an internal track system for cargo transport that is powered by motor proteins such as myosin V and myosin VI. Myosin V may also colocalize with F-actin bundles. Myosin VII and Myosin X are important for filopodial assembly and dynamics… Read more…
Myosin light chain kinase is activated by calmodulin in response to an increase in intracellular calcium. It then goes on to phosphorylate regulatory myosin light chains at residues serine 19 and threonine 18. These phosphorylations enhance the ATPase activity of actin-activated myosin and so promotes myosin-driven contraction… Read more…
The kinesin superfamily of proteins represents a large class of motor proteins that carry cargo along microtubules. Conventional kinesins move along microtubule filaments in a manner that resembles human walking. This has been described as an asymmetric ‘hand-over-hand’ mechanism where one head domain steps forward ~16.2nm whilst the other head remains stationary. For each step the head domains take, the cargo is moved 8.1nm along the length of the filament and a single ATP molecule is hydrolyzed… Read more…
Movement of myosin-X is driven by ATP hydrolysis, in a unique mechanism that resembles walking or stepping. This movement is known to occur preferentially on actin bundles rather than single actin filaments. Although it is essentially a forward movement, evidence indicates that the protein may also take side-steps. This may be carried out as a means of overcoming obstacles or defects in the track… Read more…
Tension-dependent actin polymerization and assembly of stress fibers is influenced by many factors, including differences in substrate composition, rigidity, cell membrane phospholipids, external force, as well as by strength of the connection(s) between actin filaments and the adhesion. Each of these cues converges at the level of the Rho family of GTPases and their effector substrates… Read more…
Once the bundled actin filaments in dorsal stress fibers fully interact with the transverse arc filaments, the bundles become aligned and completely ‘fuse’ to create a cohesive contractile structure that is the ventral stress fiber… Read more…
Transverse arcs filaments are thought to supply the dorsal stress fibers with filaments of mixed polarity as they are assembled; how this is achieved is relatively unknown, but based on experiments using purified components , permeabilized cells and live cells, it has been suggested that myosin bundles may recruit the filaments and facilitate polarity sorting. In line with this concept, it seems reasonable to suggest that the myosin filaments may impact the dynamic interaction between the transverse arcs and dorsal stress fibers and foster their association into ventral stress fibers… Read more…
Recent data suggests that ventral stress fibers are created by reorganizing pre-existing dorsal stress fibers and transverse arcs.Other contrasting models for the formation of ventral stress fibers not covered in this resource include annealing or fusion of short actin bundles that are associated with focal adhesions… Read more…
As the crosslinked filaments are joined together to form long bundles, transverse arcs are fortified with myosin II and the width between alternating bands of α-actinin and myosin thick filaments is equalized… Read more…
Transverse arcs appear to coalesce from short actin bundles that are generated at the lamellipodial leading edge. The assembly of myosin II into thick filaments also preferentially begins in the region immediately behind the leading edge as it retracts (even in the absence of net protrusion). The transverse arcs mature as the filaments are moved centripetally into the more stable regions of the lamellum. Transverse arcs fully evolve from end-to-end annealing of α-actinin and myosin containing bundles Read more…
Once initiated in the lamellipodium, the actin filaments are extended rapidly from their barbed ends. These are commonly situated at the leading edge, and fewer free filament ends are found in the deeper regions of the lamellipodium. There is subsequent rearward transport of the actin filaments towards the cell body. An arc usually develops from and is positioned just beneath the dorsal surface of the lamella… Read more…
Dorsal stress fibers in motile cells are formed from actin filament bundles that are initiated and extended from cell-substrate adhesions at the leading edge (aka focal complexes. Initiation is triggered by the activation of adhesion molecules, such as integrin, and the tension-dependent recruitment and activation of the Rho family of GTPases, including Rac1. Assembly then takes place, with filament elongation and condensation being the primary steps involved. Assembly of dorsal stress fibers and transverse arcs appears to be connected: transverse arcs encounter dorsal stress fibers as they are transported towards the cell body via cell-wide actin-myosin contractions… Read more…
Stress fibers are contractile in nature. By exerting and maintaining tension on the underlying substratum, they form a key element of the mechanotransduction apparatus that links the cell interior and exterior. The organization of stress fibers resembles the alternating thick actin filaments and Z bands in muscle myofibrils, however, the actin and myosin filaments in stress fibers are less regular when compared to myofibrils and they do not contract uniformly along their lengths… Read more…
Stress fibers are higher order cytoskeletal structures composed of cross-linked actin filamentbundles, and in many cases, myosin motor proteins, that span a length of 1-2 micrometers. At least 4 types of stress fibers have been identified in mammalian cells. These are dorsal stress fibers, ventral stress fibers, transverse arcs, and the recently identified perinuclear actin cap, which is an important mediator in nuclear mechanotransduction… Read more…
Typically filopodia are quite dynamic and are constantly growing or retracting. Thus, periods of stasis are often short-lived and even adhesion of the filopodial tip to a substrate will not last long before the cell pulls on the site and recruits additional components or retracts, leaving behind a thin tube of membrane. There are several events that may promote stasis in retracting filopodia. These include ligand binding to receptors on a filopodium, uncapping of the filament end or capping of unstable actin filaments… Read more…
Intermediate filaments are heterogenous, they lack polarity, have a high tensile strength and are resistant to compression, twisting and bending forces, they lack motor proteins and they have slower filament dynamics… Read more…
The soluble subunit for creating intermediate filaments is a tetramer. The tetramer is created from monomers in a stepwise fashion. First, two monomers associate via their central domains to form parallel helical coils around each other… Read more…
The tight association between protofilaments provide intermediate filaments with a high tensile strength. This makes them the most stable component of the cytoskeleton. Intermediate filaments are therefore found in particularly durable structures such as hair, scales and fingernails. The primary function of intermediate filaments is to create cell cohesion and prevent the acute fracture of epithelial cell sheets under tension… Read more…
Intermediate filaments are a primary component of the cytoskeleton, although they are not found in all eukaryotes, and are absent in fungi and plants. These filaments, which extend throughout the cytoplasm and inner nuclear membrane are composed from a large family of proteins that can be broadly grouped into five classes… Read more…
Capping proteins control access to the free barbed ends of actin filaments and is therefore a major factor affecting actin filament elongation. Capping proteins have a high affinity for barbed ends and their micromolar concentration in the cytoplasm ensures that most barbed ends are capped… Read more…
Ena/VASP proteins promote actin filament elongation by tethering actin filaments to sites of active actin assembly. Ena/VASP proteins recruit actin nucleation and initiation factors (e.g. Arp2/3 complex, formins) and promote F-actin assembly through profilin-binding… Read more…
Examples of NPF accessory proteins include Verprolin (yeast), which modulates the activity of WASp with type I myosins, to promote actin assembly by Arp2/3 complex . WASp-interacting proteins (WIPs) will also regulate the WASp activity… Read more…
Nucleation Promoting Factors (NPFs) (e.g. WASP, Scar/WAVE) modulate actin filament nucleation by bringing together actin monomers and pre-existing actin filaments, for example, during filopodial initiation where they recruit the Arp2/3 complex. NPFs compete with profilin for binding to free actin (which inhibits actin nucleotide exchange); these combined functions promote actin-filament assembly at the barbed end… Read more…
Toxins such as phalloidins, cytochalasins, latrunculin A, and jasplakinolide are naturally occurring small molecules that bind to actin and alter its polymerization… Read more…
Several factors influence actin filament length and treadmilling including ATP binding on G-actin and free ATP-G-actin concentration and the rate of ATP-G-actin assembly to the ends. The critical concentration can also be adjusted. The (-) and (+) ends have a different criticial concentration (Cc) for actin filament growth. The Cc is defined as the concentration level of free ATP-G-actin where the rate of addition is balanced by the rate of loss and no net growth occurs at that end… Read more…
In the steady state phase, actin filament dynamics enter a state of equilibrium where monomer disassembly from the (-) end and polymerization at the (+) end is balanced and maintained by a critical concentration of monomers in the cytosol. This steady state assembly and disassembly is known as ‘treadmilling’… Read more…
Profilin binds simultaneously to formin and actin monomers; this interaction tethers multiple profilin-actin complexes near the growing end of actin filaments, which promotes the processive addition of actin subunits… Read more…
Formins are also capable of actin nucleation, a process which is spatiotemporally coupled with actin disassembly. Formins nucleate and polymerize actin filaments at focal adhesions at a rate of around 0.3 µm/min… Read more…
Formins promote the elongation of pre-existing filaments by removing barbed end capping proteins and forming a sleeve around the actin subunits. Formins are also capable of actin nucleation, a process which is spatiotemporally coupled with actin disassembly…Read more…
Functionally, cortactin is involved in a wide range of cellular processes pertaining to a variety of structures. The protein is highly enriched in mature neurons and is necessary for the formation and stability of dendritic spines. Cortactin also forms part of the actin-rich core of both podosomes and invadopodia and is also involved in non-migratory cellular processes, such as the formation of cell-cell contacts… Read more…
Cortactin specifically stabilizes Arp2/3-mediated branch points along actin filaments through its repetitive actin binding sites. Although cortactin is a weak activator of the Arp2/3 complex when compared to class I NPFs (e.g. WASP, SCAR/WAVE), cortactin also binds to other NPFs (e.g. N-WASP) and their interacting proteins (e.g. WIP). This association may help to both recruit and activate Arp2/3 complex-mediated nucleation of actin filaments… Read more…
Actin filaments are highly dynamic and their polymerization is usually correlated to their disassembly. Generally, actin filament polymerization occurs over three phases: A nucleation phase, an elongation phase and a steady state phase…Read more…
Although the most commonly described nucleators are the Arp2/3 complex, and the formins, a third group, known as ‘tandem-monomer-binding nucleators’, has also been identified. Each member possesses tandem repeats of G-actin binding motifs. Included in this group of nucleators are the Spire proteins, Cordon-bleu (Cobl), Leiomodin (Lmod-2), JMY and adenomatous polyposis coli (APC)… Read more…
The Arp2/3 complex is composed of 7 evolutionarily conserved subunits (Arp2, Arp3, ARPC1-C5) that are structurally similar to the barbed end of actin [1]. The complex is inherently inactive, however once activated it facilitates the nucleation of actin monomers from existing filaments as new branches or daughter filaments… Read more…
Actin nucleation sees the formation of an actin nucleus, which is essentially a complex of three actin monomers, from which an actin filament may elongate. This process most commonly involves actin nucleators such as the Arp2/3 complex or members of the formin family of proteins… Read more…
Along with actin filament disassembly or severing, ADF/cofilin was recently shown to carry out another important role; specifically the regulation of Myosin II mediated contractility and actomyosin formation. This was proposed to result from competitive antagonism, where myosin II must compete with cofilin for binding sites on F-actin… Read more…
Mechanistically, cofilin binds between actin subunits when a longitudinal bond spontaneously breaks as the filament bends in thermal motion. Cooperative binding of ADF/cofilin causes the filament to twist and structurally weaken; this causes a modest severing effect that results in pointed end depolymerization and a 2-3 fold decrease in the average length… Read more…
Disassembly of actin filaments occurs at the pointed end of the filament and is driven by the ADF/cofilin (AC) family of proteins. Actin monomers intrinsically dissociate from the barbed end at a faster rate than they do from the pointed end… Read more…
The specific cascade of events leading to disassembly and turnover of the podosome architecture are not fully understood. Podosome disassembly is suggested to involve myosin IIA-induced contractions, affecting first the adhesive ring and then the actin core, as illustrated in dendritic cells where myosin IIA is the predominant myosin isoform… Read more…
Interactions between the extracellular matrix (ECM) and cell surface integrins leads to podosome formation. The initiating signal is transduced through mechanosensing integrins to the cytoskeleton, upon which the actin network undergoes significant re-organization to promote formation of the podosome. It is widely believed that Arp2/3-mediated nucleation is the major means by which the podosome actin cytoskeleton is built. In addition to the known activation of Arp2/3 by Cdc42-WASP, a second weaker activator of Arp2/3 also resides in podosomes (and invadopodia), namely cortactin… Read more…
Podosome initiation and assembly is highly regulated, both spatially and temporally. Dendritic cells best exemplify the temporal regulation of podosome formation. Following activation by an antigen or inflammatory cytokine, immature dendritic cells have a small window of time, roughly 6 hours, during which podosomes are able to form. Spatially the nature of the extracellular matrix (ECM) and the distribution of ligands within it, have been shown to affect the initiation of podosome assembly, as demonstrated by experiments with macrophages on fibronectin… Read more…
Podosome initiation occurs in response to interactions between ECM ligands, such as fibronectin and fibrinogen, with cell surface integrins. Distinct integrins are recruited to the adhesive ring structure of podosomes, namely integrin β2 in dendritic cells and macrophages and integrin β3 in osteoclasts… Read more…
During the process of differentiation from osteoclast precursors to mature osteoclasts, clusters of podosomes rearrange themselves into higher order rings structures, which are finally reorganized into a single belt around the cell periphery… Read more…
Both focal adhesions and podosomes are intimately involved in cell motility, with podosomes specifically implicated in cell invasion. Invasiveness is achieved through the secretion of matrix metalloproteinases (MMPs) from the core of podosomes, which degrade the extracellular matrix (ECM)… Read more…
Structurally, the podosome is characterized by two main features – an actin core and a ring complex. The actin core contains several coordinators of actin nucleation. The ring complex comprises integrins and integrin-associated proteins, such as paxillin and serves to connect cell surface integrins with the cytoskeleton… Read more…
Podosomes are actin-rich, adhesive structures that are present at the ventral surface of cells of the monocytic myeloid lineage, stimulated endothelial cells and cultured Src-transformed cancer cells. These structures are not limited to the cell periphery, but do exhibit a polarized distribution pattern in migrating cells, localizing to the front at the border between the lamellipodium and the lamellum…Read more…
Motor proteins propel themselves along the cytoskeleton using a mechanochemical cycle of filament binding, conformational change, filament release, conformation reversal, and filament rebinding. In most cases, the conformational change(s) on the motor protein prevents subsequent nucleotide binding and/or hydrolysis until the prior round of hydrolysis and release is complete… Read more…
MMPs are produced as inactive precursors which are exposed to the extracellular milieu either via secretion or translocation to the membrane. Once exposed to the extracellular environment they are activated through proteolytic cleavage of the N terminal autoinhibitory domain… Read more…
Matrix metalloproteinases (MMPs) are a family of proteases that digest components of the extracellular matrix (ECM) and surface receptors… Read more…
Along with actin filament disassembly or severing, ADF/cofilin was recently shown to carry out another important role; specifically the regulation of Myosin II mediated contractility and actomyosin formation. This was proposed to result from competitive antagonism, where myosin II must compete with cofilin for binding sites on F-actin … Read more…
Under normal circumstances, the plasma membrane remains tightly bound to the cell cortex. This close association is maintained by interactions with the actin cytoskeleton, myosin and other associated proteins. Hydrostatic pressure is exerted on the plasma membrane via cortical tension generated by myosin . However, during blebbing, myosin contracts the cortical actin cytoskeleton, detaching it from the plasma membrane… Read more…
Circus movements have been mainly observed in embryonic blastomeres, neuroectoderm and paraxial mesoderm , where a single bleb repeatedly propagates around the circumference of the cell with a period of 1–2 minutes…Read more…
Retraction generally lasts between 60 to 120 seconds . When the cells membrane lacks the stability afforded, for example, by cell-substrate contacts, the primary cascade involved in retraction is that of mysoin-RhoA-ROCK …Read more…
As expansion of the bleb begins to slow, cortical actin just beneath the bleb membrane begins to repolymerize. This mechanims of actin nucleation is unclear, as two of the most common actin nucleaters, the Arp2/3 complex and the formin mDia1, are not present beneath the membrane of filamin-deficient cells … Read more…
In motile blebbing, cells move by exerting a force against the underlying substrate. In lamellipodial motility, this is achieved through adherence of lamellipodia to the substrate. However, in blebbing motility, the mechanism is still unknown. Two models have been proposed : Weak substrate adhesion and perpendicular force generation… Read more…
Motile blebbing (as opposed to non-motile) occurs primarily at the leading edge. The stimulus and subsequent downstream signaling that initiates this polarization is not yet clear. However, two polarization models have been proposed, one based on the local membrane detachment (1) and the other based on the local cortex rupture scenario (2) … Read more…
Expansion lasts between 5 to 30 seconds, following bleb initiation and preceding reformation of the cortical actomyosin cytoskeleton just beneath the membrane . During this time, the bleb proceeds to grow as a result of actomyosin driven pressure, resulting in the influx of cytoplasm into the bleb… Read more…
Blebs are blister-like protrusions that occur at the cell surface (reviewed in ). Blebs form, and function, in a series of defined steps. They typically grow to a length of around 2 µm within 30 seconds, before shrinking back for another 120 seconds. Blebs are well known as a by-product of apoptotic and necrotic processes, even though they are not essential for the execution of either of these programs . In recent decades, the role that blebs play in the locomotion of some cell types became increasingly acknowledged. For example, bleb-mediated cell motility was observed in early embryos and was termed amoeboid motility …Read more…
GTP hydrolysis has been shown to be a key regulator of microtubule polymerization dynamics. Although the exact mechanisms are poorly understood, two opposing models have been proposed to describe how GTP could alter the conformation of α-tubulin/β-tubulin from the intrinsically ‘bent spring’ shape that resists straightening, to the straight conformation that gives rise to microtubule stability… Read more…
Although most microtubule growth and shrinkage occurs at the plus end, it can also occur at the minus end. Certain proteins sever and break microtubules… Read more…
In most cell types, thirteen protofilaments associate laterally to form a microtubule. In a few cases microtubules contain more or fewer protofilaments . Numerous interactions between the subunits give microtubules their stiffness and resistance to bending forces… Read more…
Microtubule proliferation has also been shown to increase the intracellular viscosity of myoctyes and impede sarcomere shortening, which is required to maintain contractility of the cardiac muscle … Read more…
Microtubules exist in all cells, however their influence in the mechanotransduction of mechanical stimuli has been described at length in cardiac striated muscle …Read more…
There are 4 main functions of microtubules:
1.To form an architectural framework that establishes the overall polarity of the cell
2. To form the spindle apparatus and ensure the proper segregation of duplicated chromosomes into daughter cells during cell division
3.To form an internal transport network for the trafficking of vesicles containing essential materials to the rest of the cell.
4. To form a rigid internal core that is used by microtubule-associated motor proteins to generate force and movement in motile structures such as cilia and flagella.
Microtubules are hollow cylinders that are approximately 25nm in diameter and vary in length from 200 nm to 25 μm. They are formed by the lateral association of between 12 and 17 protofilaments into a regular helical lattice ,… Read more…
Crosslinking of the filaments by specific motors or multivalent binding proteins (accessory proteins) increases stability and forms higher-order structures.
Examples of higher-order cytoskeleton structures include contractile bundles (muscle cells), the microtubule organizing center (MTOC), the nuclear lamina and the intermediate filament-based ‘cage’ that forms around the nucleus from flexible cables at the cell surface to the center of the cell… Read more…
During neural development, highly motile structures on the developing neurites, called growth cones, are guided by signals from the extracellular envrionment. Guidance cues come in many different forms, from diffusible extracellular proteins and lipid factors, to extracellular matrix proteins and/or carbohydrates located on the cell substrate… Read more…
Growth cones facilitate the growth and guidance of axons by bundling and extending actin filaments into structures known as filopodia and microspikes. Binding of filopodia and adhesion receptors to particular extracellular matrix (ECM) components or ligands is translated into actin filament assembly, cytoskeleton remodeling and force-driven motility. These events culminate in the growth of the neuron towards its target… Read more…
Growth cone collapse is a complex phenomenon involving numerous signal pathways including Rho-GTPases , ADF , and various kinases ,… Read more…
A number of factors regulate collapse and retraction. For example, capping proteins promote filopodial retraction by shielding the barbed end of filaments from further assembly and elongation … Read more…
Binding of filopodia to certain ligands or substratum may hinder filament assembly, thereby leading to changes that promote retraction, collapse or growth cone turning … Read more…
Although a reterograde motion of actin filaments is intrinsic in the formation of filopodia, the forces generated by actin treadmilling are too weak to facilitate the “pulling” mechanism required for rigidity sensing and other mechanosensing processes. This characteristic of filopodia is instead produced by the activity of myosin motor proteins such as Myosin II … Read more…
Basal adhesions play a specific role in filopodia initiation and are found in ~98% of all filopodia, where they anchor the filopodial base that usually remains immobile despite considerable flexibility in the shaft . These are stable adhesions that contain a focal ring structure believed to convert tension forces into filopodia formation… Read more…
A single filopodium can have both non-adherent and adherent regions along the shaft. Shaft adhesions develop de novo along the filopodium and do not represent former tip adhesions … Read more…
Filopodia on apposed cells interact directly through their tips and/or ‘slide’ past each other (interdigitate) in order for adhesions to form (through cadherin-cadherin contact) between the tip of one filopodia and the cell membrane at the base of the adjacent filopodium … Read more…
A diverse array of cellular responses can result when a filopodium makes contact with a ligand or substrate. These responses are dependent on the coupling of membrane-bound proteins to the backward (retrograde) flow of actin that drives filopodia elongation and motility. Each adhesion may function independently or work in concert to produce the overall guidance response. The three types of adhesions that form within filopodia are: Tip adhesions, shaft adhesions and basal adhesions… Read more…
Typically filopodia are quite dynamic and are constantly growing or retracting. Thus, periods of stasis are often short-lived and even adhesion of the filopodial tip to a substrate will not last long before the cell pulls on the site and recruits additional components or retracts, leaving behind a thin tube of membrane. There are several events that may promote stasis in retracting filopodia… Read more…
Filopodia are motile structures, being able to extend, retract and move laterally as they sense their environment. Lateral movement is particularly important in allowing the structure to sense stimuli prior to its adhesion with another cell or substrate… Read more…
The extension rate of a filopodium will differ depending on the cell type (table 1). In each case however, this rate is controlled by the availability of G-actin-ATP, associated structural components and the energetics of membrane bending… Read more…
As well as mediating cargo transport along actin filament bundles, recent studies have implicated myosin-X as integral to the initiation of filopodia and the elongation of long filopodia. This has been attributed to a mechanism of myosin-X that promotes actin filament bundling, similar to cross-linking… Read more…
Filopodia protrusion is aided by actin filament cross-linking, which gives the structure the strength required to push against the compressive force of the plasma membrane . Bundle stiffness increases with the number of bundled filaments and so contributes to the overall length of the filopodium . Filament bundling results from crosslinking proteins, many of which co-localize at the base of filopodia and work in concert to produce crosslinked filaments … Read more…
Once nucleation has taken place, actin filaments begin to extend. Actin filaments in filopodia are unbranched , indicating filaments elongate primarily through the activity of formins, however numerous other proteins also play important roles… Read more…
Knockdown of Arp2/3 in cultured neurons as well as loss-of-function mutations in C. elegens and cultured Drosophila neurons, was shown to cause a disruption in filopodia initiation and subsequently a decrease in the number of filopodia… Read more…
The first step in the formation of a filopodium is the nucleation of actin filaments from G-actin monomers. This is facilitated by various proteins known as nucleators, and may occur via the ‘tip nucleation model’ or the ‘convergent nucleation model’… Read more…
Filopodia are dynamic structures that are primarily composed of F-actin bundles and whose initiation and elongation are precisely regulated by the rate of actin filament assembly, convergence and cross-linking… Read more…
Filopodia (singular filopodium) are thin membrane protrusions that act as antennae for a cell to probe the surrounding environment ,,… Read more…
For forces to be translated into a net forward gain in cellular movement, the trailing edge must retract as the leading edge protrudes forward. In order for this to occur focal adhesions at the rear of the cell, and the actin filament network to which they are linked, must be disassembled. Prevention of this step would result in the cell being permanently anchored to its substrate… Read more…
Interactions between actin filament networks and the focal adhesions to which they are linked results in the generation of forces. These forces may be exerted internally through actin bundle tension and filament network dynamics or externally as the cell pushes on its surroundings. A number of studies, discussed below, have focused on measuring the protrusive forces generated by lamellipodia … Read more…
Experiments have demonstrated that a biphasic relationship exists between the rate of actin flow and traction stress . Whilst they are inversely related in the lamellipodium where nascent adhesions are formed and actin flow rate is high, the relationship becomes linear in areas with larger adhesions and slow actin flow , generating maximal propulsion at intermediate flow rates … Read more…
Focal adhesions essentially act as “molecular clutches”, promoting protrusion at the leading edge whilst suppressing membrane contraction (reviewed in ). Adhesions aid forward movement by regulating the forces produced by actin dynamics in different cellular compartments through several methods… Read more…
Although the lamellipodial actin network is highly dynamic, moments of pause and stasis have been reported … Read more…
Filament extension occurs via the ‘actin treadmilling’ mechanism, with lamellipodial growth reflecting the balance between actin filament polymerization at the barbed ends and retrograde actin flow towards the cell body (reviewed in )…. Read more…
Extension of newly formed actin filament branches occurs at the interface between the leading edge of the lamellipodia, and the existing actin filament network and is maintained by mechanisms such as actin treadmilling… Read more…
Current evidence suggests multiple nucleators may function alongside the Arp2/3 complex , although the extent to which they influence the growth of the actin filament network and its ability to exert a protrusive force on the cell membrane remains unclear… Read more…
In the first phase of lamellipodia formation, actin filament polymerization produces a protrusive force on the cell membrane that promotes the spreading out and enlargement of the lamellipodia. In polarized, migrating cells this is known as the leading edge… Read more…
Although there are numerous details that remain unresolved, it is abundantly clear that mechanical mechanisms are essential for coordinating the physical and biochemical processes that determine cell shape and locomotion… Read more…
The actin cytoskeleton plays an essential role in the formation and function of the lamellipodia. Lamellipodial actin filaments are highly dynamic, especially compared to those of the lamella and it is due to their dynamic nature, and the constant cycles of actin filament polymerization and actin filament depolymerization that the protrusive force required to stretch the membrane and allow the lamellipodia to spread, is generated… Read more…
In the traditional model, when adhesions are strong and the leading edge is anchored to the substrate, the cell pulls itself against the adhesion and moves forward in the process. The lamellipodial actin network continues to be pulled backwards, over the lamella, until it is severed from the initial leading edge adhesion. The generation of new actin filaments ensures lamellipodial growth and protrusion continues and new adhesions can be formed …In the alternative model where actin filaments are gathered in the lamellipodia as ‘arcs’ and pulled back into the lamella by Myosin II, adhesions, and adhesion strength, play important roles in the rate of migration… Read more…
Traditionally, it is believed that the lamellipodia and lamella are composed of two distinct actin networks , with the more dynamic lamellipodial actin lying on top of, and moving over, the more stable lamellal actin network . A more recent model suggests maturing actin filaments, in the form of bundled ‘arcs’, form in the lamellipodia and are drawn back into the existing more stable actin filament network of the lamella… Read more…
The lamellipodia and lamella are plate-like extensions of the cell that play crucial roles in both cell motility and migration, and mechanosensing. These structures form and function over distinct steps… Read more…
The final step in function of invadopodia is disassembly which primarily involves dismantling the actin core . Several proteins have been implicated in a cascade leading to this, including paxillin, extracellular signal-regulated kinases (Erk) and calpain …Read more…
The main function attributed to invadopodia is that of extracellular matrix (ECM) degradation, facilitated by the secretion of proteases… Read more…
The rate of invadopodia extension is dependent upon the ability of the growing filaments to overcome membrane resistance and the concomitant incorporation of new membrane. This can be achieved through the addition of membrane from vesicles delivered to sites of invadopodia formation… Read more…
Elongation of invadopodia is suggested to be driven by the extension of parallel arrays of bundled actin filaments that are present along the length of invadopodia . Extension of these filaments is driven by formins, whilst their bundling is coordinated by the actin cross-linker fascin… Read more…
Following initiation by an appropriate signal, the actin cytoskeleton is reorganized to facilitate invadopodia formation. Two distinct types of filamentous actin networks are suggested to cooperatively form invadopodia – a branched actin network that forms the base of the structure and a parallel array of bundled actin filaments that form along the length of the invadopodial shaft… Read more…
Initiation of invadopdia formation is highly complex, being influenced by various signalling cascades and phosphorylation events that occur following detection of a stimulant… Read more…
The basic structure of an invadopodium consists of a F-actin core similar to that found in podosomes. Microtubules and intermediate filaments have also been detected in mature invadopodia … Read more…
Although invadopodia are similar to podosomes in many respects and share many common protein constituents, there are several key differences between these organelles … Read more…
Invadopodia are actin-rich structures that are present at the basal surfaces of cells capable of crossing extracellular barriers, such as cancer cells. The primary function of invadopodia appears to be the focal degradation of the extracellular matrix (ECM) through the secretion of matrix metalloproteinases (MMPs) ,. The formation and function of invadopodia can be described over defined steps that include initiation, extension, ECM degradation and disassembly… Read more…
Proteins containing I-BAR (inverted Bin/amphiphysin/Rvs i.e. IRSp53 Missing-in-metastasis homology Domain or IMD) cooperate with various components of actin filament assembly, to promote filopodia protrusion, via several mechanisms including the stimulation of F-actin crosslinking … Read more…
α-actinin primarily influences the cohesiveness and mechanics of the cytoskeleton by cross-linking actin filaments and other cytoskeleton components to create a scaffold that imparts stability and forms a bridge between the cytoskeleton and signaling pathways… Read more…
α-actinin is an actin-binding protein and component of the actin crosslinking functional modules; it lacks G-actin binding activity and lacks actin initiation/nucleation activity . α-actinin is an important organizer of the cytoskeleton that belongs to the spectrin superfamily (which includes spectrin, dystrophin, and related homologues)… Read More…
Fimbrin (aka plastin homologue, accumentin) is an actin binding protein that was originally identified in microvilli.
Fimbrin represents one of the most basic structures of an actin crosslinking protein;… Read more…
Filamin forms a vital scaffolding adaptor and regulatory component that contributes to the mechanical stability of cells by linking the internal actin network with membrane receptors and mechanosensitive components. This function correlates with its distribution in cultured cells along actin stress fibers, within cortical actin networks and sometimes at membrane ruffles… Read more…
Filamin is an actin-binding protein that was first recognized (and named) for its filamentous colocalization with actin stress fibers. Filamin molecules are naturally found in cells as elongated, V-shaped dimers (i.e. two linked filamin molecules) that contain several immunoglobulin-like domains for protein interactions, amino-terminal actin-binding domains (ABD) composed of two calponin homology (CH) domains, and a carboxy-terminal dimerization domain … Read more…
A steady pool of F-actin monomers or loosely linked F-actin promotes efficient polymerization and bundling of actin-filaments by fascin.
Fascin is found in the most distal regions of filopodia and lamellipodia and the cellular distribution of fascin within actin bundles (e.g. microspikes and stress fibers ) appears to vary depending upon the extracellular substrate… Read more…
Fascin is the major actin crosslinking protein found in a wide range of filopodia. This protein has been shown to work in concert with other cross linkers such as α-actinin to produce filopodia, although fascin itself is sufficient to form filopodia-like bundles in a reconstitution system…Read more…
Crosslinking of actin filaments is facilitated by actin binding proteins such as a-actinin, fascin or filamin. These proteins tether actin filaments together to strengthen the cytoskeleton, and enable their arrangement into higher order actin-based structures… Read More
The eukaryotic cytoskeleton is a network of three long filament systems – microtubules, actin filaments and intermediate filaments. These networks are made from the dynamic assembly and disassembly of protein components. The cytoskeleton creates an internal architecture to give a cell its shape through elaborate linkage(s) to itself, the plasma membrane, and internal organelles. The cytoskeleton structure is modified by adhesion to neighboring cells or to the extracellular matrix (ECM)… Read more…
Actin filaments and their associated focal adhesion complexes act as information handling machines or mechanosensors: they convert both the strength of the adhesion and the tensile forces along the linked network of actin filaments (and associated proteins) into biochemical signals that control actin extension and cell migration… Read more…
The orientation of individual actin filaments in the cytoskeleton is a force-driven evolutionary process that contributes to the elastic behavior of the network and influences whether a filament will deform by compression, bending or extension. Cross-linked actin networks initially become more elastic under low force as a result of filament resistance to the direction of compression. As the force increases, individual filaments inherently resist being compressed and/or cross-linking proteins become more extended, which causes the cytoskeleton network to become more rigid; cell stiffening has also been correlated with actin recruitment… Read more…
Factors that influence the concentration of free G-actin (e.g. profilin [40]) or ATP-binding and hydrolysis on actin will promote filament assembly and membrane protrusion. Furthermore, the microtubule and intermediate filament networks also play a key role in regulating the global deposition pattern of the actin filaments and therefore also influence membrane protrusion dynamics… Read more…
Actin filaments are initiated with their barbed ends oriented towards the plasma membrane, with ATP hydrolysis facilitating filament growth. Polymerization is favored towards the cell front and disassembly occurs more frequently at the rear. However, only a small fraction of the overall free energy of nucleotide hydrolysis is needed to modulate G-actin monomer binding. The remaining energy is translated into a protrusive force that deforms the plasma membrane in a particular direction… Read more…
Troponin, a three-peptide complex, is thought to trap tropomyosin in a calcium-dependent fashion at a position that inhibits myosin bundles from accessing the actin filaments; calcium binding to troponin allows a conformational restructuring of tropomyosin that leaves the myosin-binding sites on the thin filaments exposed…Read more…
Actin-based motile structures are disassembled before cell division, which causes the cell to stop moving and become more rounded. More stable actin bundles remain polarized and contribute to the orientation of the microtubule network that serves as the mitotic spindle. The proper assembly, orientation, and contraction of an actin filament ring (i.e. contractile ring) serves to pinch and separate the daughter cells during cell division…. Read more…
Actin filaments are the primary cytoskeletal component to drive cell motility. Actin filaments found in membrane protrusions such as filopodia and lamellipodia rapidly assemble and disassemble. These cellular structures are essential in cell migration and are predominately found at the leading edge of a moving cell. They also allow the cell to probe or sense its microenvironment… Read more…
Actin filaments are crucial for tissue organization and for establishing cell polarity and cohesion among epithelial cells. For example, a core of actin filaments provides microvilli structural support and enables them to increase their surface area and nutrient-absorbing capacity. These structures are found on the apical surface of epithlial cells lining the small intestine…Read more…
The polarity of an actin filament is visualized by the binding of the myosin subfragment (S1) to the filament, which creates barbed (+) and pointed (-) ends on the filament. When all actin subunits are bound by myosin S1, the filament appears coated with arrowheads that all point towards one end of the filament… Read more…
Actin filaments (F-actin) are linear polymers of globular actin (G-actin) subunits and occur as microfilaments in the cytoskeleton and as thin filaments, which are part of the contractile apparatus, in muscle and nonmuscle cells. They commonly underlie the plasma membrane and are typically assembled at the cell periphery from adhesion sites or sites of membrane extension… Read more…
Non-muscle myosin II isoforms have a similar structure and function to their muscle equivalents. However, their interaction with actin serves to generate cellular forces rather than muscular contraction… Read more…
In non-muscle cells, myosin II associates with actin filaments to form contractile structures known as stress fibers along the lower surfaces where the cell is anchored to its substrate. In epithelial cells, contractile bundles are also prominent in the adhesion belt (aka adherens belt), which helps to maintain the stability and integrity of epithelial cell sheets. The contractile bundles in nonmuscle cells are similar to skeletal muscle fibers, but they are smaller (~0.4 µm in fibroblasts), less organized, and they contain different accessory proteins … Read more…
In skeletal muscle cells, myosin II forms only thick filaments that are arranged within a scaffold of actin thin filaments (along with numerous other proteins). These form the higher order fibrous structures known as sarcomeres… Read more…
Each member of the myosin family possesses unique structural and functional properties, such as their step size, that determines their ability to engage in F-actin translocation . It has been shown that myosins in general are required for this process to facilitate filopodial retraction … Read more…
Actomyosin refers to the actin-myosin complex that forms within the cytoskeleton. Actomyosin is inherently contractile, with the myosin motor protein able to pull on actin filaments. This property gives rise to contractile fibers that form the basis of skeletal muscle, and even in non-muscle cells, enable cell motility and force generation at the sub-cellular level… Read more…
Contractile force can be inhibited using small molecules such as blebbistatin . This is a cell-permeable, highly specific small-molecule inhibitor of myosin II Mg-ATPase activity that is used for investigating the role of myosin II in cell contraction and motility. Blebbistatin inhibits both myosin isoforms IIA, IIB, and skeletal muscle myosin II but has little effect on smooth muscle myosin II and myosins I, Myosin V, and Myosin X … Read More…
Contractile bundles vary in thickness and have been shown to contain anywhere between 10 to 300 individual actin filaments … Read More…
Contractile fibers are intracellular protein filament-based structures that are primarily composed of actin, myosin and tropomyosin… Read more…
Actin filaments may be assembled with, and stabilized by, accessory proteins into higher order contractile structures such as stress fibers (nonmuscle cells) or contractile bundles (muscle cells). The dynamic association of tropomyosin and troponin with actin filaments stabilizes the actin filament (collectively termed “thin filament”) to be functional in various contexts… Read More…
- What is the cytoskeleton?
- What are actin filaments?
- What are motor proteins?
- What is Myosin?
- What steps are involved in the myosin powerstroke?
- What are contractile fibers?
- What is actomyosin?
- How does cofilin regulate actomyosin formation and Myosin II mediated contractility?
- How does the contractome protein network regulate actomyosin contractility?
- How is polarity established by cellular forces?Sruthi Jagannathan2018-06-13T14:59:32+08:30
How is polarity established by cellular forces?
- How are apoptotic cells removed from the epithelial tissue in Drosophila pupae?Sruthi Jagannathan2018-04-16T14:41:11+08:30
How are apoptotic cells removed from the epithelial tissue in Drosophila pupae?
- What is the role of non-junctional E-cadherin clusters?Sruthi Jagannathan2018-03-12T12:57:36+08:30
What is the role of non-junctional E-cadherin clusters?
- How do geometric constraints alter cell shape and rearrangement in curved epithelial tissues?Sruthi Jagannathan2018-03-01T12:45:29+08:30
How do geometric constraints alter cell shape and rearrangement in curved epithelial tissues?
- What is the role of basolateral cell protrusions in driving Drosophila germ-band extension?steve2018-02-19T11:10:53+08:30
What is the role of basolateral cell protrusions in driving Drosophila germ-band extension?
- What is the role of DAAM1 at actin nodes?Andrew Wong2018-01-29T15:40:12+08:30
What is the role of DAAM1 at actin nodes?
- What is the role of plastin in C. elegans embryogenesis?steve2018-02-08T13:15:06+08:30
What is the role of plastin in C. elegans embryogenesis?
References
- Luchi RJ, and Kritcher EM. Drug effects on cardiac myosin adenosine triphosphatase activity. J. Pharmacol. Exp. Ther. 1967; 158(3):540-5. [PMID: 4294509]
- Langanger G, Moeremans M, Daneels G, Sobieszek A, De Brabander M, and De Mey J. The molecular organization of myosin in stress fibers of cultured cells. J. Cell Biol. 1986; 102(1):200-9. [PMID: 3510218]
- Woolley DE. An actin-like protein from amoebae of dictyostelium discoideum. Arch. Biochem. Biophys. 1972; 150(2):519-30. [PMID: 4261413]
- Spudich JA. Biochemical and structural studies of actomyosin-like proteins from non-muscle cells. II. Purification, properties, and membrane association of actin from amoebae of Dictyostelium discoideum. J. Biol. Chem. 1974; 249(18):6013-20. [PMID: 4278010]
- Kreis TE, and Birchmeier W. Stress fiber sarcomeres of fibroblasts are contractile. Cell 1980; 22(2 Pt 2):555-61. [PMID: 6893813]
- Katoh K, Kano Y, Masuda M, Onishi H, and Fujiwara K. Isolation and contraction of the stress fiber. Mol. Biol. Cell 1998; 9(7):1919-38. [PMID: 9658180]