Read Further…
For a complete introduction to the role of actin in mechanotransduction see: How Actin Filaments Produce, Sense and Convert Cellular Force’
Actin is an essential component in Filopodia, Lamellipodia and Lamellum, Podosomes and Adherens junctions
Actin is an abundant (10-100 micromolar on average),~42 kDa structural protein found in all eukaryotic cells (except for nematode sperm). With more than 95% conservation in the primary structure, actin is one of the most highly-conserved proteins [1]. G-actin is the monomeric, globular form of actin that forms the basic subunit for actin filaments. It is believed to fold into an elongated structure that consists
of a large and a small domain which together form a cleft that binds ATP [2, 3].
Polymerization
Actin monomers are assembled to form actin filaments or ‘thin filaments’.
The polymerization of actin filaments, requires ATP-bound G-actin. Assembly of G-actin-ATP is favored at the (+) end of actin filaments. Hydrolysis of ATP to ADP and the steady release of Pi on G-actin cause a conformational change which favors the disassembly of G-actin-GDP at the (-) end of actin filaments. The main actin-binding proteins in vertebrate cells, profilin
and thymosin-β4, control the available pool of monomeric G-actin and
inhibit spontaneous nucleation of actin filaments. Although actin bound
to thymosin-β4 fails to polymerize, profilin can compete with thymosin
for actin binding and can shuttle actin to the barbed-end of a filament [4].
Early models for the actin filament were constructed by fitting the filament x-ray crystal structure to the atomic structure of actin monomers [5] (reviewed in [6]) while more recent models use a number of different approaches [7, 8]. Collectively these results suggest that when single actin strands form, two asymmetric actin monomers align to form a twofold axis of symmetry [9]; their subsequent assembly into a filament that is composed of a pair of strands causes a left-handed helical twist when the adjacent subunits are positioned with respect to each other [10].
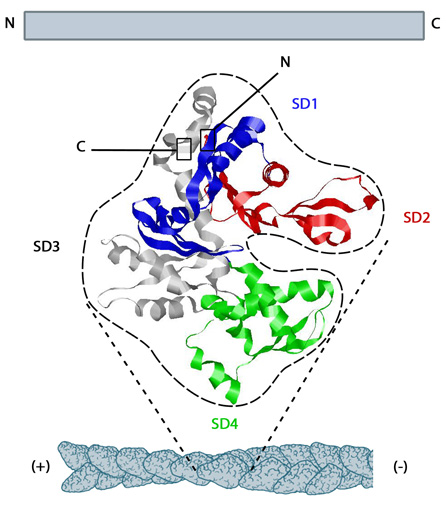
Figure: Structure of G-actin and its assembly into filaments. The structure shown here [3] was downloaded from the RSCB Protein Data Bank (PDB file: 1atn). The ATP binding cleft is starred (*) on the right. Actin comprises four subdomains, termed SD1 (blue), SD2 (red), SD3 (grey) and SD4 (green). The barbed end of each monomer (SD1 and SD3) is shown on the left and the pointed end (SD2 and SD4) is shown on the right. Similarly the polarity of the actin filament, which comprises these monomers is shown at the bottom, with the barbed (+) end on the left and the pointed (-) end on the right.