Read Further…
For more information read about myosin motor proteins read: Myosin Isoforms; Motor Proteins; The “Powerstroke” Cycle; Contractile bundles and Thick filaments
Myosin is a motor protein that uses the energy from ATP hydrolysis to move along actin filaments.
Many myosin isoforms have been found in eukaryotes (See Figure below), which differ in the type of heavy and light chains they are composed of. All myosins are composed of a diverse ‘tail’ domain at their carboxy terminus and an evolutionarily conserved globular ‘head’ domain at their amino terminus.
The diverse ‘tails’ of different myosin isoforms bind specific substrates or cargo, whilst their conserved ‘heads’ contain sites for ATP binding [1], F-actin binding and force generation (i.e. motor domains) (reviewed in [2, 3]).
All myosins bind to actin filaments via a globular ‘head’ domain located at the end of the heavy chains. Actin binding to this region increases the ATPase activity of myosins (reviewed in [2][4]. Some myosins have a single heavy chain and contact actin filaments at only one site, while other myosin isoforms have two heavy chains and contact actin filaments at two sites. Myosin II is the only family member that can form polymeric assemblies[3]) (See “thick filaments” below).
The number of light chains influences the length of the “lever arm” or “neck region” and therefore the “step size” of different myosin types [5]. Myosin V contains more light chains relative to myosin II and so myosin V moves in larger steps along actin filaments after an equivalent round of ATP hydrolysis (reviewed in [6]).
Myosin motors have directionality on actin filaments, with all myosins moving towards the barbed end, except myosin VI that moves towards the pointed end. Most actin filaments have the barbed end directed towards the plasma membrane and the pointed end towards the interior. This arrangement allows certain myosins (e.g. myosin V) to function primarily for cargo export, while myosin VI acts as the major motor protein for import. Myosin II is commonly associated with retraction fibers and retrograde actin flow at the pointed end of actin filaments. All non-muscle cells use contractile bundles containing myosin II to generate forces that promote the assembly of actin filaments.
Although most myosins function as motor proteins in the cytoplasm, some species of myosin are localized to, and function in, the nucleus. Nuclear Myosin I (NMI) myosin II, myosin V, myosin VI, myosin XVIB and myosin XVIIIB have all been found in the nucleus [7, 8, 9], with NMI being the most extensively studied.
 Figure: The myosin superfamily of motor proteins. All
myosins share a motor domain on their heavy chains at the
amino-terminus (the ‘head’ domain), but they differ considerably at
their carboxy-terminus (the ‘tail’ domain). A few myosin types also
have an amino-terminal extension. The number of light chains varies
considerably between myosin types and certain myosins exist as dimers.
Myosins that form dimers have two motor domains, and the number of light
chains can influence the “lever arm” length
between the myosin heads – this regulates the length of the myosin
‘powerstroke’ and the distance the myosin can travel along the actin
filament in a single round of ATP hydrolysis (see also ‘myosin
powerstroke’).
Figure: The myosin superfamily of motor proteins. All
myosins share a motor domain on their heavy chains at the
amino-terminus (the ‘head’ domain), but they differ considerably at
their carboxy-terminus (the ‘tail’ domain). A few myosin types also
have an amino-terminal extension. The number of light chains varies
considerably between myosin types and certain myosins exist as dimers.
Myosins that form dimers have two motor domains, and the number of light
chains can influence the “lever arm” length
between the myosin heads – this regulates the length of the myosin
‘powerstroke’ and the distance the myosin can travel along the actin
filament in a single round of ATP hydrolysis (see also ‘myosin
powerstroke’).
The diverse ‘tails’ of different myosin isoforms bind specific substrates or cargo, whilst their conserved ‘heads’ contain sites for ATP binding [1], F-actin binding and force generation (i.e. motor domains) (reviewed in [2, 3]).
All myosins bind to actin filaments via a globular ‘head’ domain located at the end of the heavy chains. Actin binding to this region increases the ATPase activity of myosins (reviewed in [2][4]. Some myosins have a single heavy chain and contact actin filaments at only one site, while other myosin isoforms have two heavy chains and contact actin filaments at two sites. Myosin II is the only family member that can form polymeric assemblies[3]) (See “thick filaments” below).
The number of light chains influences the length of the “lever arm” or “neck region” and therefore the “step size” of different myosin types [5]. Myosin V contains more light chains relative to myosin II and so myosin V moves in larger steps along actin filaments after an equivalent round of ATP hydrolysis (reviewed in [6]).
Myosin motors have directionality on actin filaments, with all myosins moving towards the barbed end, except myosin VI that moves towards the pointed end. Most actin filaments have the barbed end directed towards the plasma membrane and the pointed end towards the interior. This arrangement allows certain myosins (e.g. myosin V) to function primarily for cargo export, while myosin VI acts as the major motor protein for import. Myosin II is commonly associated with retraction fibers and retrograde actin flow at the pointed end of actin filaments. All non-muscle cells use contractile bundles containing myosin II to generate forces that promote the assembly of actin filaments.
Although most myosins function as motor proteins in the cytoplasm, some species of myosin are localized to, and function in, the nucleus. Nuclear Myosin I (NMI) myosin II, myosin V, myosin VI, myosin XVIB and myosin XVIIIB have all been found in the nucleus [7, 8, 9], with NMI being the most extensively studied.
 Figure: The myosin superfamily of motor proteins. All
myosins share a motor domain on their heavy chains at the
amino-terminus (the ‘head’ domain), but they differ considerably at
their carboxy-terminus (the ‘tail’ domain). A few myosin types also
have an amino-terminal extension. The number of light chains varies
considerably between myosin types and certain myosins exist as dimers.
Myosins that form dimers have two motor domains, and the number of light
chains can influence the “lever arm” length
between the myosin heads – this regulates the length of the myosin
‘powerstroke’ and the distance the myosin can travel along the actin
filament in a single round of ATP hydrolysis (see also ‘myosin
powerstroke’).
Figure: The myosin superfamily of motor proteins. All
myosins share a motor domain on their heavy chains at the
amino-terminus (the ‘head’ domain), but they differ considerably at
their carboxy-terminus (the ‘tail’ domain). A few myosin types also
have an amino-terminal extension. The number of light chains varies
considerably between myosin types and certain myosins exist as dimers.
Myosins that form dimers have two motor domains, and the number of light
chains can influence the “lever arm” length
between the myosin heads – this regulates the length of the myosin
‘powerstroke’ and the distance the myosin can travel along the actin
filament in a single round of ATP hydrolysis (see also ‘myosin
powerstroke’). Thick filament
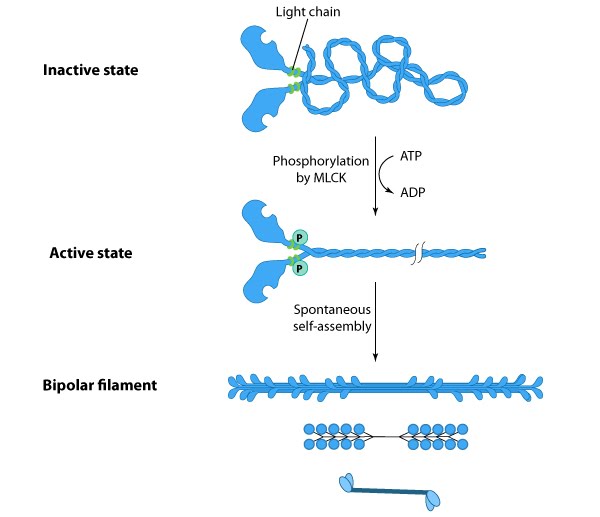
Certain myosin isoforms (i.e. myosin II) form higher order assemblies via the extended
coiled-coil domains in the heavy chains. Myosin II filaments in
fibroblasts are dispersed throughout the cell except for the very
leading edge [10]. Myosin II bundle formation and ATPase activity in
nonmuscle cells is regulated by phosphorylation [10, 11] (reviewed in
[12, 13, 14, 15, 16][10].
Phosphorylation of myosin light chains (MLC) by myosin light chain kinase (MLCK) or Rho-associated kinases allows the long coiled-coil
domains of myosin II to interact in a cooperative manner with the
coiled-coiled domains of adjacent myosin II molecules, followed by
additional tail-tail interactions with other myosin II assemblies; the
resulting bipolar myosin II bundle is called a ‘”thick filament” (see
figure below). MLC phosphorylation not only precedes actin
polymerization and stress fiber formation [17] but it also influences the
intracellular localization of myosin II [18]. Unphosphorylated myosin
can still form filaments (in vitro[10].
Figure: Formation of thick filaments. This diagram depicts how phosphorylation of myosin II promotes bundle formation and gives examples for how the resulting bipolar thick filaments are illustrated throughout this resource.
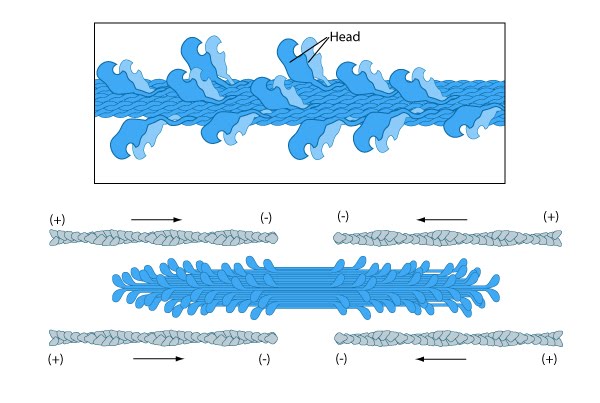
Bipolar thick filaments have several hundred myosin heads oriented in opposite directions at the two ends of the filament. Concerted ATP hydrolysis and movement of the myosin heads along adjacent actin thin filaments generates a sliding motion that results in shortening or contraction of the interlinked actin filaments.
Figure: Formation of thick filaments. This diagram depicts how phosphorylation of myosin II promotes bundle formation and gives examples for how the resulting bipolar thick filaments are illustrated throughout this resource.
Bipolar thick filaments have several hundred myosin heads oriented in opposite directions at the two ends of the filament. Concerted ATP hydrolysis and movement of the myosin heads along adjacent actin thin filaments generates a sliding motion that results in shortening or contraction of the interlinked actin filaments.
Figure: Thick filament structure.