Read Further…
Explore how zyxin contributes to Focal adhesion maturation and Fibrillar adhesion
Read more on the related cytoskeleton scaffolding protein Tensin.
Protein structure
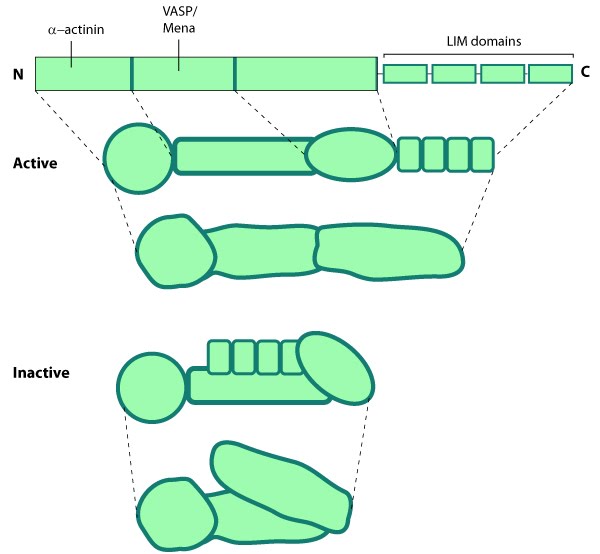
Zyxin, meaning a joining [1], is an important scaffolding protein and component of the actin linking functional module. Zyxin has a proline-rich amino-terminus and a carboxy-terminus containing three LIM domains which are recognized historically as zinc-binding motifs (see figure below) [2]. The LIM domains of zyxin have been shown to bind to factors that control gene transcription [3] and they function as regulatory domains for controlling protein-protein interactions with components involved in cell-cell junction assembly [4, 5]. Zyxin is also thought to be autoregulated by an intramolecular interaction between the LIM domains and the VASP binding site [5].Figure: Zyxin. This schematic diagram illustrates the molecular organization of zyxin [5] and provides examples for how zyxin is represented in figures throughout this resource. Relevant domains believed to be important for protein-protein interactions are highlighted: VASP [6]; FA targeting [7], α-actinin [8]; zyxin also binds Vav in the N-terminal proline-rich region [9].
Localization and function
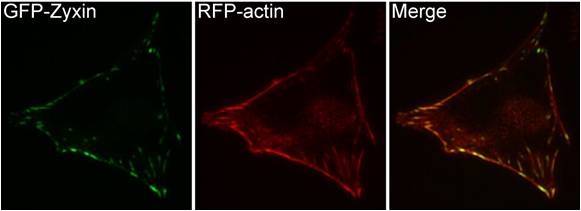
Zyxin is enriched along actin filaments, stress fiber bundles, and at cell-cell or cell-matrix adhesion sites (see figure below) [1, 2]. Zyxin is specifically found in more mature adhesions [10] and its absence in early adhesions is commonly used to distinguish the ‘age’ of an adhesion [11]. The main function of zyxin is to form a bridge between the adhesion components at the cell membrane and the internal cytoskeleton (reviewed in [12]). Zyxin is vital for coordinating matrix-dependent cues with actin dynamics; for example, within stress fibers and focal adhesions (FAs), zyxin acts as a mechanosensor that binds to areas where forces are applied [9-11]. Not only is zyxin binding proportional to the mechanical force (e.g. decreased traction reduces zyxin-binding) but its stability at adhesion sites is tension-dependent [13, 14].Cellular adaptation to mechanical stress also involves redistribution of zyxin from FAs to stress fibers, which causes stress fiber thickening [15]. Mislocalization of zyxin leads to defects in cell migration and spreading [16, 6] and its absence leads to increased cellular motility [17] presumably through reduced adhesive strength [18]. Zyxin influences actin organization and assembly around FAs by recruiting Ena/VASP [6, 17, 7]; Ena/VASP may subsequently generate new actin filaments by an unknown mechanism that is initiation factor-independent, however, this has not been demonstrated in intact cells [19].
Figure. Zyxin cellular localization. A NIH3T3 cell, plated on a collagen coated glass slide and transfected with RFP-actin and GFP-zyxin. The basal surface of the cell was imaged using TIRF microscopy, on an Olympus IX81 Inverted microscope at 60x magnification. [Image captured by Machiyama Hiroaki, Mechanobiology Institute, Singapore]