Protein Structure
Ena/VASP (vasodilator-stimulated phophoprotein) belongs to a group of highly related multifunctional actin-binding proteins that includes Drosophila Enabled (Ena), its mammalian homologue Mena (mammalian Enabled), the murine homologue Evl (Enabled/vasodilator-stimulated phosphoprotein-like), and the C. elegans homologue unc34.
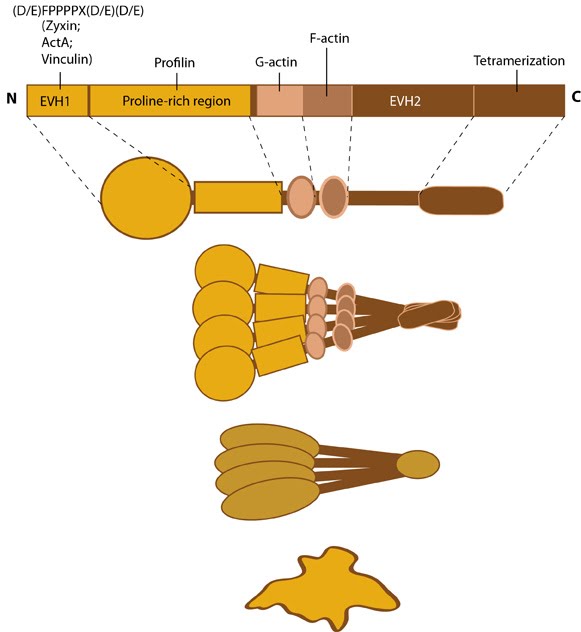
Proteins of the Ena/VASP family share a common structural organization
composed of highly conserved amino and carboxy terminal domains (called
Ena-VASP homology 1 and 2 [EVH1 and EVH2]); these domains mediate
binding to actin filament nucleation factors (e.g. bacterial ActA, formins) and focal adhesion proteins (e.g. zyxin, vinculin)
(reviewed in [1]). Members of the Ena/VASP family are thought to
oligomerize in solution and be active as a tetramer in cells (see Figure
below) [2, 3]. Phosphorylation of VASP proteins alters their ability
to bind to actin filaments but not their ability to form a tetramer or
interact with known ligands (e.g. profilin, vinculin, zyxin) [4].
Figure: Ena/VASP family. This schematic diagram illustrates the molecular organization of the Ena/VASP protein family and provides examples for how the Ena/VASP proteins are represented in figures throughout this resource. Relevant domains believed to be important for binding to actin and for protein-protein interactions are highlighted (reviewed in [1]).
Ena/VASP localization
Proteins of the Ena/VASP family contribute to cell movement, axon guidance, neural tube closure and shape change in vertebrate cells by modulating actin filament organization and dynamics; these effects are achieved in part by regulating the morphology and behavior of actin-based structures such as lamellipodia and filopodia
(reviewed in [1]). Ena/VASP proteins also modulate actin dynamics at
sites of cell-ECM and cell-cell interactions and they are concentrated
to the proximal portion of phosphotyrosine-rich domains at the ends of
F-actin stress fibers [5].
Ena/VASP function
Ena/VASP proteins promote
actin filament elongation by tethering the filaments to sites of active
actin assembly [6, 7, 8]. Ena/VASP proteins recruit actin nucleation and
initiation factors (e.g. Arp2/3 complex, formins) and they promote G-actin
assembly through profilin-binding (reviewed in [9]). The rate of actin
filament elongation by Ena/VASP proteins is determined in by the
recruitment of G-actin via teh G-actin binding site (GAB) that lies
within the EVH2 domain and shares close sequence homology to WASP
homology 2 motifs [10]. Ena/VASP proteins are also thought to accumulate
at the plasma membrane where they alter actin polymerization by
antagonizing barbed (+) end capping proteins,
thereby enabling the incorporation of actin into longer filaments
[6, 7]; however, controversy over their exact mechanism still exists
(reviewed in [1]). In addition, Ena/VASP may promote actin assembly by
an unknown mechanism that is independent of initiation factors, however,
this has not been demonstrated in intact cells [11]
Ena/VASP activity in filopodia:
The Ena/VASP family promotes filopodia
formation through cooperation with formins and by modulating actin
filament assembly [12] (reviewed in [13]). Ena/VASP protects actin
filaments from capping, thereby enhancing filament elongation and
promoting F-actin bundling, which together help stimulate filopodium
protrusion [8, 14, 15, 16, 17]. VASP sustains filopodia protrusion and extension
through its localization at the filopodia tips [18]; myosin X is
responsible for this transport of VASP to the tip [19]. Formins
are found at adhesion junctions and formin-mediated actin assembly may
play a part in extending the contact area after cell-cell adhesion of
filopodia on apposed cells [20]. Knowing that VASP tethers growing actin
filaments to the filopodium tip [7], implies that VASP may couple actin
assembly and actin rearward movement to membrane protrusion, thereby
expanding the region of cell-cell contact.
Ena/VASP activity at adhesions:
Ena/VASP proteins associate with components of the
Arp2/3-mediated actin assembly module and they are required for actin
dynamics at sites of cadherin-cadherin binding [21]. The association of
Mena and VASP may be modulated by signal-mediated phosphorylation
(reviewed in [22]); VASP phosphorylation prevents it from interacting
with other cadherin-complex proteins (e.g. zyxin) [23].
Ĉ ď Steven Wolf, Jan 17, 2012, 7:06 PM Ĉ ď Steven Wolf, Jan 17, 2012, 7:06 PM
|