Additional Links
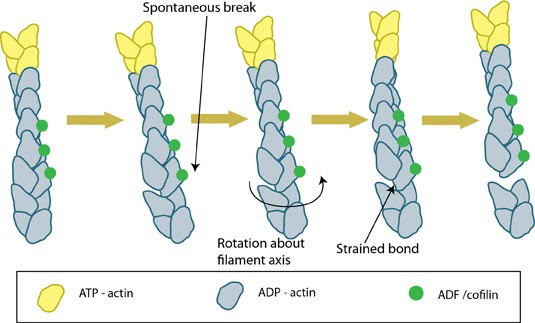
Essential for : Actin Treadmilling in Filopodia and Lamellipodia formation. Retraction of the Trailing Edge Influenced by : Cross linking of actin bundles | Functional Module: ADF/Cofilin in Actin Filament DisassemblyWhole cell motility and mechanosensing rely on the continual restructuring of the cytoskeleton, particularly within lamellipodia and filopodia; two dynamic structures that contribute to cell motility. The turnover of actin filaments within these structures maintains a pool of actin monomers that permits the continual restructuring and growth of the actin cytoskeleton.Disassembly of actin filaments occurs at the pointed end of the filament and is driven by the ADF/cofilin (AC) family of proteins. Actin monomers intrinsically dissociate from the barbed end at a faster rate than they do from the pointed end [1]. This is counteracted by the binding of capping proteins or formins to the barbed end, creating a more stable filament. The action of cofilin at the pointed end serves to destabilize the filament and promote the release of ADP-actin monomers. The conversion of ATP-F-actin to ADP-F-actin involves the hydrolysis of ATP and subsequent release of free inorganic phosphate (Pi) molecules. These free Pi molecules bind antagonistically to cofilin and as such cofilin binding to F-actin precedes Pi release [2]. Whilst Pi stabilizes the actin filament once bound, AC proteins destabilize the filament by inducing conformational change. This involves the production or stabilization of a twist within the filament or between monomers, creating a strain that leads to the loss of filament integrity and disassembly. The conformational change induced by cofilin binding further promotes filament destabilization through increasing the rate of Pi release by approximately 10-fold [2]. The pH of the environment also affects the ability of cofilin to depolymerize actin filaments, with a higher pH favoring depolymerization due to the weaker binding of Pi in more alkali environments [3]. Recent data suggests that the efficacy of cofilin in filament severing is enhanced in actin bundles despite slower binding kinetics of cofilin to fascin-bundled actin filaments. This enhanced efficacy permits the the severing of bundled actin filaments at concentrations of cofilin that are inadequate to induce severing of free filaments. It was proposed that this is the result of cross-linkers such as fascin reducing the flexibility of the actin filaments and subsequently making them more vulnerable to the twisting effect of cofilin; as the filament as a whole is unable to provide slack for the sections under pressure [4]. The destabilized form of actin filaments, which has been compared to that observed in younger filaments [5], is more prone to filament severing. The higher affinity of ACs for ADP-F-actin relative to ATP-F-actin causes severing in the central regions of filaments where ADP-actin is enriched, though depolymerization at the pointed end also occurs [6]. Differences between ADF and Cofilin It should be noted that the AC proteins have been shown to initiate nucleation of new filaments from recently disassembled monomers. In this case, cofilin shows a higher nucleating activity (double) compared to ADP [3]. Based on this finding, which was observed at pH8 in experimental conditions, ADF is considered to be more effective in promoting actin turnover as the free monomers are less likely to undergo ADF mediated nucleation immediately following disassembly [3]. Figure: ADF/cofilin severs actin filaments. Actin filaments are held together by bonds between both lateral and longitudinal actin subunits. Spontaneous breaks in the longitudinal (weaker) bonds occurs as a result of movement caused by Brownian motion; these breaks can be reformed unless ADF/cofilin binds to the actin at the breakpoint and prevents re-forming of the longitudinal bonds. ADF-binding causes additional rotation and produces strain on the surrounding filament bonds; this strain increases the rate at which the filament is fully severed. (Figure adapted from [7]). A role in Actomyosin ContractionLatest Findings Along with actin filament disassembly or severing, ADF/cofilin was recently shown to carry out another important role; specifically the regulation of Myosin II mediated contractility and actomyosin formation. This was proposed to result from competitive antagonism, where myosin II must compete with cofilin for binding sites on F-actin [8].
In this study it was shown that the binding affinities of each protein are ATP dependent, with ADF/cofilin possessing a competitive advantage at cellular levels of ATP, whilst in the absence of ATP the binding affinities of each protein is similar. Importantly, a reduction in the levels of both ADF and cofilin lead to an increase in the concentration of F-actin, a finding that was attributed not to a loss in cofilin mediated F-actin severing, but rather to an increase in myosin-II dependent actin assembly via its crosslinking properties. This was confirmed with the introduction of blebbistatin which inhibited myosin II activity and subsequently lead to the disassembly of F-actin [8]. The implications for this role of ADF/cofilin may be described at the molecular level, however as shown by Wiggan O et al the consequences are clearly evident at a cellular level, with persistent membrane blebs being observed in HeLa cells depleted of the proteins [8]. As it had previously been reported that non-apoptotic blebs were produced as a means of releasing cell tension, it is probable that the observed phenotype occurred for a similar purpose, and highlights the importance of ADF/cofilin in the regulation of cortical tension and actomyosin activity [8]. Despite these findings, in some situations a cooperative relationship between ADF/cofilin and Myosin-II appears to exist. This has been described, for example, in a study investigating actomyosin ring constriction in budding yeast cells [9]. Supporting earlier findings [10, 11], this study also confirmed that deletion or inhibition of the motor-domain of Myosin II (MyoI) did not completely prevent constriction, but noted that a 40% reduction in the rate of contraction was observed. This was in contrast to mutations in cofilin, or stabilization of actin filaments, which did prevent actomyosin ring constriction. Model simulations using this data indicated a role of Myosin-II in the promotion of cofilin mediated depolymerization, and it was suggested that it is the disassembly of F-actin that is the primary contributor to actomyosin ring constriction [9]. |
References
- Pollard TD. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J. Cell Biol. 1986; 103(6 Pt 2):2747-54. [PMID: 3793756]
- Muhlrad A., Pavlov D., Peyser YM. & Reisler E. Inorganic phosphate regulates the binding of cofilin to actin filaments. FEBS J. 2006; 273(7):1488-96. [PMID: 16689934]
- Yeoh S., Pope B., Mannherz HG. & Weeds A. Determining the differences in actin binding by human ADF and cofilin. J. Mol. Biol. 2002; 315(4):911-25. [PMID: 11812157]
- Breitsprecher D., Koestler SA., Chizhov I., Nemethova M., Mueller J., Goode BL., Small JV., Rottner K. & Faix J. Cofilin cooperates with fascin to disassemble filopodial actin filaments. J. Cell. Sci. 2011; 124(Pt 19):3305-18. [PMID: 21940796]
- Bernstein BW. & Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 2010; 20(4):187-95. [PMID: 20133134]
- Carlier MF., Laurent V., Santolini J., Melki R., Didry D., Xia GX., Hong Y., Chua NH. & Pantaloni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J. Cell Biol. 1997; 136(6):1307-22. [PMID: 9087445]
- Maciver SK., Zot HG. & Pollard TD. Characterization of actin filament severing by actophorin from Acanthamoeba castellanii. J. Cell Biol. 1991; 115(6):1611-20. [PMID: 1757465]
- Wiggan O., Shaw AE., DeLuca JG. & Bamburg JR. ADF/cofilin regulates actomyosin assembly through competitive inhibition of myosin II binding to F-actin. Dev. Cell 2012; 22(3):530-43. [PMID: 22421043]
- Mendes Pinto I., Rubinstein B., Kucharavy A., Unruh JR. & Li R. Actin depolymerization drives actomyosin ring contraction during budding yeast cytokinesis. Dev. Cell 2012; 22(6):1247-60. [PMID: 22698284]
- Fang X., Luo J., Nishihama R., Wloka C., Dravis C., Travaglia M., Iwase M., Vallen EA. & Bi E. Biphasic targeting and cleavage furrow ingression directed by the tail of a myosin II. J. Cell Biol. 2010; 191(7):1333-50. [PMID: 21173112]
- Lord M., Laves E. & Pollard TD. Cytokinesis depends on the motor domains of myosin-II in fission yeast but not in budding yeast. Mol. Biol. Cell 2005; 16(11):5346-55. [PMID: 16148042]