Additional Links
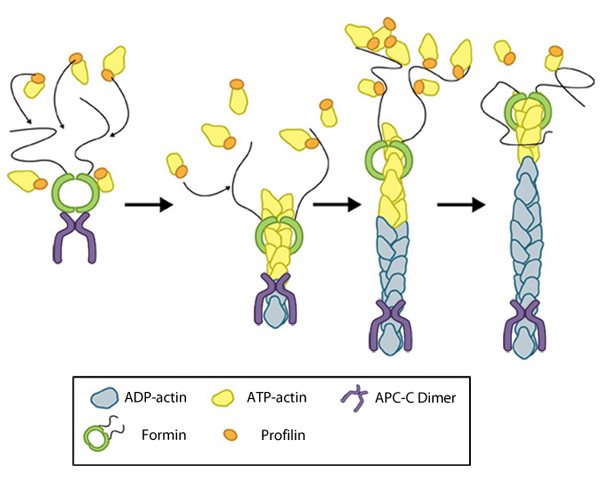
Essential for: Initiation in Filopodia Formation Initiation in Lamellipoda and Lamella Focal adhesion initiation Podosome extension Related in Function to: Arp2/3 Mediated Nucleation | Functional Module: Formin and Profilin in Actin NucleationActin filament assembly and disassembly are primary molecular processes that facilitate whole cell motility and the movement of subcellular structures. The initial stage in the formation of actin filaments is nucleation. This is defined as the formation of a stable actin polymer from its monomeric units [1].Although nonmuscle cells have a high concentration of G-actin-ATP (~100 μM) [2], pure G-actin monomers fail to nucleate new actin filaments efficiently due to the instability of actin oligomers. Additional factors are therefore necessary for the production of actin filaments. Several models of actin nucleation have been described [1], including formin-mediated nucleation, which involves a number of proteins, particularly members of the formin family, profilin and G-actin. Members of the formin family of proteins (that includes mDia) are key regulators of unbranched actin filament initiation and nucleation (reviewed in [3]). They are essential for generating, extending and/or protecting lamellipodial actin filaments from capping [4] and are also also key regulators of actin bundle initiation and formation in filopodia [5, 6] and stress fibers [7, 8, 9]. The formin, mDia2, is suggested to be involved in both initiation of invadopodia formation through actin nucleation and subsequent growth of invadopodia through the elongation of actin filaments [10]. Formins are essential in the nucleation of new filaments, as they stabilize actin dimers (reviewed in [11]). Under normal circumstances they are auto-inhibited through structural interactions between the two ends of the protein [12]. However, conformational rearrangements resulting in their activation can be induced through interactions with GTP-bound (active) Rho GTPases [13]. This process remains poorly understood (reviewed in [3]). Latest Findings In a recent study, the tumor suppressor adenomatous polyposis coli (APC) was shown to bind the formin mDia1 and overcome capping protein- and profilin-mediated suppression of spontaneous actin nucleation, resulting in the initiation of actin filament nucleation and elongation [14]. In the mechanism described, APC is primarily responsible for actin monomer recruitment, whilst mDia1 catalyzes filament elongation. Actin recruitment by APC did not involve capturing F-actin intermediates that had spontaneously formed nor did APC contribute to filament elongation. In this model, once actin polymerization commences the APC-mDia1 complex separates – mDia1 is propelled away from APC along with the growing barbed end of the filament and APC remains attached to the filament at the site of nucleation [14]. Although a consensus has yet to be reached for the mechanism of formin-mediated nucleation, it is now well-established that activated formins function as dimers and form a donut-shaped complex around terminal actin subunits, orientating themselves toward the (+) end of the actin filament or nucleus [15]. This binding is facilitated by FH2 (formin homology 2) domains within the formin monomers. Next, each formin monomer binds and captures profilin units, which are themselves already bound to G-actin monomers. This interaction is mediated by multiple stretches of polyproline residues within the FH1 domain of formins [16]. This domain is known to range from 15-229 residues, consist of between 35% and 100% proline residues, and contain up to 16 profilin binding sites [17]. Profilin maintains a steady pool of actin monomers by promoting ADP to ATP nucleotide exchange on G-actin [15]. These monomers of ATP-G-actin are then added the growing actin filament. The coupling of formin with the growing end prevents capping and allows continued growth of the filaments [18]. Figure: Formin-mediated nucleation of actin filaments. The FH2 domains of the formin dimer (shown in green) bind to actin monomers to initiate filament assembly. Recent studies indicate this is assisted, or even mediated, by additional factors such as APC. The FH1 domains of the formin dimer (shown as black lines) have short polyproline sequences that interact with profilin. Profilin binds to both formin and actin monomers to increase the addition of actin monomers to the barbed end of the filament. |
References
- Firat-Karalar EN. & Welch MD. New mechanisms and functions of actin nucleation. Curr. Opin. Cell Biol. 2011; 23(1):4-13. [PMID: 21093244]
- Rosenblatt J., Peluso P. & Mitchison TJ. The bulk of unpolymerized actin in Xenopus egg extracts is ATP-bound. Mol. Biol. Cell 1995; 6(2):227-36. [PMID: 7787248]
- Chesarone MA., DuPage AG. & Goode BL. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat. Rev. Mol. Cell Biol. 2010; 11(1):62-74. [PMID: 19997130]
- Yang C., Czech L., Gerboth S., Kojima S., Scita G. & Svitkina T. Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 2007; 5(11):e317. [PMID: 18044991]
- Schirenbeck A., Bretschneider T., Arasada R., Schleicher M. & Faix J. The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat. Cell Biol. 2005; 7(6):619-25. [PMID: 15908944]
- Pellegrin S. & Mellor H. The Rho family GTPase Rif induces filopodia through mDia2. Curr. Biol. 2005; 15(2):129-33. [PMID: 15668168]
- Gasteier JE., Madrid R., Krautkrämer E., Schröder S., Muranyi W., Benichou S. & Fackler OT. Activation of the Rac-binding partner FHOD1 induces actin stress fibers via a ROCK-dependent mechanism. J. Biol. Chem. 2003; 278(40):38902-12. [PMID: 12857739]
- Koka S., Neudauer CL., Li X., Lewis RE., McCarthy JB. & Westendorf JJ. The formin-homology-domain-containing protein FHOD1 enhances cell migration. J. Cell. Sci. 2003; 116(Pt 9):1745-55. [PMID: 12665555]
- Gupton SL., Eisenmann K., Alberts AS. & Waterman-Storer CM. mDia2 regulates actin and focal adhesion dynamics and organization in the lamella for efficient epithelial cell migration. J. Cell. Sci. 2007; 120(Pt 19):3475-87. [PMID: 17855386]
- Schoumacher M., Goldman RD., Louvard D. & Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J. Cell Biol. 2010; 189(3):541-56. [PMID: 20421424]
- Dominguez R. Structural insights into de novo actin polymerization. Curr. Opin. Struct. Biol. 2010; 20(2):217-25. [PMID: 20096561]
- Li F. & Higgs HN. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr. Biol. 2003; 13(15):1335-40. [PMID: 12906795]
- Watanabe N., Madaule P., Reid T., Ishizaki T., Watanabe G., Kakizuka A., Saito Y., Nakao K., Jockusch BM. & Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997; 16(11):3044-56. [PMID: 9214622]
- Breitsprecher D., Jaiswal R., Bombardier JP., Gould CJ., Gelles J. & Goode BL. Rocket launcher mechanism of collaborative actin assembly defined by single-molecule imaging. Science 2012; 336(6085):1164-8. [PMID: 22654058]
- Romero S., Le Clainche C., Didry D., Egile C., Pantaloni D. & Carlier MF. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell 2004; 119(3):419-29. [PMID: 15507212]
- Paul AS., Paul A., Pollard TD. & Pollard T. The role of the FH1 domain and profilin in formin-mediated actin-filament elongation and nucleation. Curr. Biol. 2008; 18(1):9-19. [PMID: 18160294]
- Higgs HN. Formin proteins: a domain-based approach. Trends Biochem. Sci. 2005; 30(6):342-53. [PMID: 15950879]
- Pring M., Evangelista M., Boone C., Yang C. & Zigmond SH. Mechanism of formin-induced nucleation of actin filaments. Biochemistry 2003; 42(2):486-96. [PMID: 12525176]