Additional Links Modulators of integrin activation Essential for: Focal adhesion (FA) initiation Podosomes Lamellipodia Related links: Integrins Talin | Functional Module: Activators and Integrin
Integrin activation is an important mechanism through which cells
regulate integrin function by manipulating the ligand affinity of
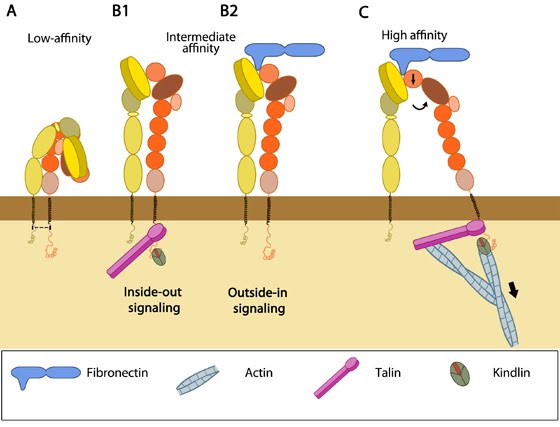
integrins spatially and temporally. Structural and functional studies suggest that integrins can exist in different ligand affinity states- low, intermediate and high (reviewed in [1]). Crystal
structures have revealed that integrin heterodimers,
occur in an inactive, bent V-shape with the head close to the
membrane-proximal regions of the legs [2, 3], maintained by the α/β salt bridge at the inner membrane region and helix packing of the transmembrane (TM) region [4]. This low affinity structure undergoes rapid,
reversible conformational changes to increase ligand affinity, termed “activation” (reviewed in [5, 6, 7]). |
References
- Luo BH., Carman CV. & Springer TA. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007; 25:619-47. [PMID: 17201681]
- Xiong JP., Stehle T., Diefenbach B., Zhang R., Dunker R., Scott DL., Joachimiak A., Goodman SL. & Arnaout MA. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science 2001; 294(5541):339-45. [PMID: 11546839]
- Xiong JP., Stehle T., Zhang R., Joachimiak A., Frech M., Goodman SL. & Arnaout MA. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science 2002; 296(5565):151-5. [PMID: 11884718]
- Partridge AW., Liu S., Kim S., Bowie JU. & Ginsberg MH. Transmembrane domain helix packing stabilizes integrin alphaIIbbeta3 in the low affinity state. J. Biol. Chem. 2005; 280(8):7294-300. [PMID: 15591321]
- Shimaoka M., Takagi J. & Springer TA. Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct 2002; 31:485-516. [PMID: 11988479]
- Calderwood DA. Integrin activation. J. Cell. Sci. 2004; 117(Pt 5):657-66. [PMID: 14754902]
- Banno A. & Ginsberg MH. Integrin activation. Biochem. Soc. Trans. 2008; 36(Pt 2):229-34. [PMID: 18363565]
- Takagi J., Petre BM., Walz T. & Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 2002; 110(5):599-11. [PMID: 12230977]
- Puklin-Faucher E., Gao M., Schulten K. & Vogel V. How the headpiece hinge angle is opened: New insights into the dynamics of integrin activation. J. Cell Biol. 2006; 175(2):349-60. [PMID: 17060501]
- Tadokoro S., Shattil SJ., Eto K., Tai V., Liddington RC., de Pereda JM., Ginsberg MH. & Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science 2003; 302(5642):103-6. [PMID: 14526080]
- Shattil SJ., Kim C. & Ginsberg MH. The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 2010; 11(4):288-300. [PMID: 20308986]
- Zhu J., Luo BH., Xiao T., Zhang C., Nishida N. & Springer TA. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol. Cell 2008; 32(6):849-61. [PMID: 19111664]
- Takada Y., Ye X. & Simon S. The integrins. Genome Biol. 2007; 8(5):215. [PMID: 17543136]
- Watanabe N., Bodin L., Pandey M., Krause M., Coughlin S., Boussiotis VA., Ginsberg MH. & Shattil SJ. Mechanisms and consequences of agonist-induced talin recruitment to platelet integrin alphaIIbbeta3. J. Cell Biol. 2008; 181(7):1211-22. [PMID: 18573917]
- Han J., Lim CJ., Watanabe N., Soriani A., Ratnikov B., Calderwood DA., Puzon-McLaughlin W., Lafuente EM., Boussiotis VA., Shattil SJ. & Ginsberg MH. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr. Biol. 2006; 16(18):1796-806. [PMID: 16979556]
- Calderwood DA., Yan B., de Pereda JM., Alvarez BG., Fujioka Y., Liddington RC. & Ginsberg MH. The phosphotyrosine binding-like domain of talin activates integrins. J. Biol. Chem. 2002; 277(24):21749-58. [PMID: 11932255]
- Wegener KL., Partridge AW., Han J., Pickford AR., Liddington RC., Ginsberg MH. & Campbell ID. Structural basis of integrin activation by talin. Cell 2007; 128(1):171-82. [PMID: 17218263]
- Anthis NJ., Wegener KL., Ye F., Kim C., Goult BT., Lowe ED., Vakonakis I., Bate N., Critchley DR., Ginsberg MH. & Campbell ID. The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J. 2009; 28(22):3623-32. [PMID: 19798053]
- Saltel F., Mortier E., Hytönen VP., Jacquier MC., Zimmermann P., Vogel V., Liu W. & Wehrle-Haller B. New PI(4,5)P2- and membrane proximal integrin-binding motifs in the talin head control beta3-integrin clustering. J. Cell Biol. 2009; 187(5):715-31. [PMID: 19948488]
- Kim M., Carman CV. & Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 2003; 301(5640):1720-5. [PMID: 14500982]
- Moser M., Nieswandt B., Ussar S., Pozgajova M. & Fässler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 2008; 14(3):325-30. [PMID: 18278053]
- Pluskota E., Dowling JJ., Gordon N., Golden JA., Szpak D., West XZ., Nestor C., Ma YQ., Bialkowska K., Byzova T. & Plow EF. The integrin coactivator kindlin-2 plays a critical role in angiogenesis in mice and zebrafish. Blood 2011; 117(18):4978-87. [PMID: 21378273]
- Harburger DS., Bouaouina M. & Calderwood DA. Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. J. Biol. Chem. 2009; 284(17):11485-97. [PMID: 19240021]
- Nilsson S., Kaniowska D., Brakebusch C., Fässler R. & Johansson S. Threonine 788 in integrin subunit beta1 regulates integrin activation. Exp. Cell Res. 2006; 312(6):844-53. [PMID: 16405888]
- Karaköse E., Schiller HB. & Fässler R. The kindlins at a glance. J. Cell. Sci. 2010; 123(Pt 14):2353-6. [PMID: 20592181]
- Moser M., Legate KR., Zent R. & Fässler R. The tail of integrins, talin, and kindlins. Science 2009; 324(5929):895-9. [PMID: 19443776]
- Rossier O., Octeau V., Sibarita JB., Leduc C., Tessier B., Nair D., Gatterdam V., Destaing O., Albigès-Rizo C., Tampé R., Cognet L., Choquet D., Lounis B. & Giannone G. Integrins β1 and β3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat Cell Biol. 2012; 14(10):1057-67. [PMID: 23023225]
- Banno A., Goult BT., Lee H., Bate N., Critchley DR. & Ginsberg MH. Subcellular localization of talin is regulated by inter-domain interactions. J Biol Chem. 2012; 287(17):13799-812. [PMID: 22351767]
- Zhu J., Carman CV., Kim M., Shimaoka M., Springer TA. & Luo BH. Requirement of alpha and beta subunit transmembrane helix separation for integrin outside-in signaling. Blood 2007; 110(7):2475-83. [PMID: 17615290]
- Arnaout MA., Mahalingam B. & Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 2005; 21:381-410. [PMID: 16212500]
- Arias-Salgado EG., Lizano S., Sarkar S., Brugge JS., Ginsberg MH. & Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc. Natl. Acad. Sci. U.S.A. 2003; 100(23):13298-302. [PMID: 14593208]
- Law DA., DeGuzman FR., Heiser P., Ministri-Madrid K., Killeen N. & Phillips DR. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature 1999; 401(6755):808-11. [PMID: 10548108]
- Datta A., Huber F. & Boettiger D. Phosphorylation of beta3 integrin controls ligand binding strength. J. Biol. Chem. 2002; 277(6):3943-9. [PMID: 11723131]
- Miyamoto S., Akiyama SK. & Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science 1995; 267(5199):883-5. [PMID: 7846531]
- Arias-Salgado EG., Lizano S., Shattil SJ. & Ginsberg MH. Specification of the direction of adhesive signaling by the integrin beta cytoplasmic domain. J. Biol. Chem. 2005; 280(33):29699-707. [PMID: 15937333]
- Anthis NJ., Haling JR., Oxley CL., Memo M., Wegener KL., Lim CJ., Ginsberg MH. & Campbell ID. Beta integrin tyrosine phosphorylation is a conserved mechanism for regulating talin-induced integrin activation. J. Biol. Chem. 2009; 284(52):36700-10. [PMID: 19843520]
- Ginsberg MH., Partridge A. & Shattil SJ. Integrin regulation. Curr. Opin. Cell Biol. 2005; 17(5):509-16. [PMID: 16099636]
- Gahmberg CG., Fagerholm SC., Nurmi SM., Chavakis T., Marchesan S. & Grönholm M. Regulation of integrin activity and signalling. Biochim. Biophys. Acta 2009; 1790(6):431-44. [PMID: 19289150]
- Lefort CT., Hyun YM., Schultz JB., Law FY., Waugh RE., Knauf PA. & Kim M. Outside-in signal transmission by conformational changes in integrin Mac-1. J. Immunol. 2009; 183(10):6460-8. [PMID: 19864611]
- Adair BD., Xiong JP., Maddock C., Goodman SL., Arnaout MA. & Yeager M. Three-dimensional EM structure of the ectodomain of integrin {alpha}V{beta}3 in a complex with fibronectin. J. Cell Biol. 2005; 168(7):1109-18. [PMID: 15795319]
- Fagerholm SC., Hilden TJ., Nurmi SM. & Gahmberg CG. Specific integrin alpha and beta chain phosphorylations regulate LFA-1 activation through affinity-dependent and -independent mechanisms. J. Cell Biol. 2005; 171(4):705-15. [PMID: 16301335]
- Fagerholm SC., Varis M., Stefanidakis M., Hilden TJ. & Gahmberg CG. alpha-Chain phosphorylation of the human leukocyte CD11b/CD18 (Mac-1) integrin is pivotal for integrin activation to bind ICAMs and leukocyte extravasation. Blood 2006; 108(10):3379-86. [PMID: 16857989]
- Friedland JC., Lee MH. & Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science 2009; 323(5914):642-4. [PMID: 19179533]
- Ivaska J. Unanchoring integrins in focal adhesions. Nat Cell Biol. 2012; 14(10):981-3. [PMID: 23033047]