Additional Links
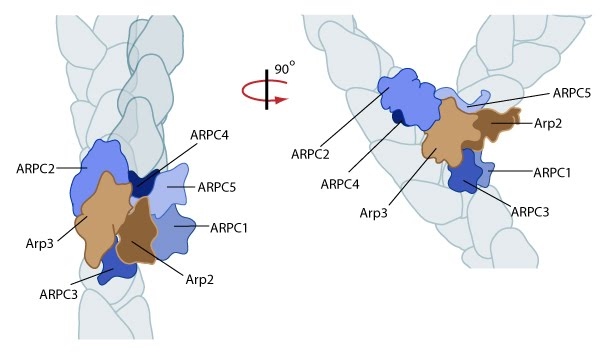
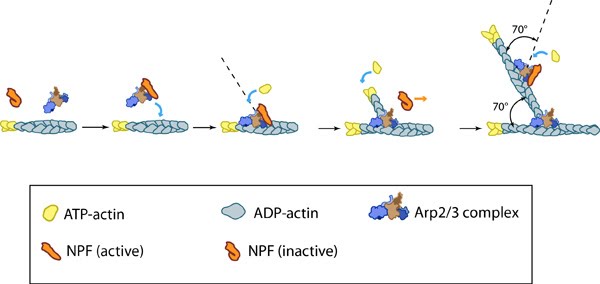
Essential for: Related in Function to: | Functional Module: Arp2/3-mediated NucleationActin filament assembly and disassembly are primary molecular processes that facilitate whole cell motility and the movement of subcellular structures.The initial stage in the formation of actin filaments is nucleation. This is defined as the formation of a stable actin polymer from its monomeric units [1]. Although nonmuscle cells have a high concentration of G-actin-ATP (~100 μM) [2], pure G-actin monomers fail to nucleate new actin filaments efficiently due to the instability of actin oligomers. Additional factors are therefore necessary for the production of actin filaments. Video: Arp2/3-mediated Actin Nucleation. The process by which the Arp2/3 complex mediates actin nucleation is shown here. This process is regulated by proteins such as WASp and Cdc42, which are depicted in this animation as stylized schematic models. [Video produced by MBInfo] The Arp2/3 complex is composed of 7 evolutionarily conserved subunits (Arp2, Arp3, ARPC1-C5) that are structurally similar to the barbed end of actin [3]. The complex is inherently inactive, however once activated it facilitates the nucleation of actin monomers from existing filaments as new branches or daughter filaments (reviewed in [1]). The Arp2/3 complex is essential in the formation of lamellipodia (LP), where it facilitates nucleation exclusively at the leading edge (as opposed to regions further away from the front). Although it is absent from the shafts of filopodia [4], it is known to play a critical role in initiation and growth of these structures [5]. Conversely, the Arp2/3 complex is present along the length of shafts of invadopodia. It has also been detected at the base of invadopodia, whilst being absent from the tips [6]. The Arp2/3 complex binds to the sides and pointed ends of pre-existing actin filaments [6], in a step which contributes to its activation [1]. Binding to nucleation promotion factors (NPFs) occurs via central/acidic (CA) sequences and is also essential in the activation of the Arp2/3 complex. Generally, cells contain a large number of inactive NPFs. Stimuli such as chemoattractants influence signaling pathways to regulate the local concentration of active NPFs near the cell membrane [7, 8, 9] (reviewed in [10]). A variety of proteins engaged in these signaling pathways are able to regulate NPF activation, such as the Rho GTPase Cdc42, which regulates the NPFs WAVE and WASp. Alternatively, NPFs can be auto-regulated by intramolecular interactions between the WCA (WASP homology 2-Connector-Acidic) and other upstream domains of the NPF, as is the case with WASp and N-WASp [1]. Figure: Reconstruction of Arp2/3 complex-mediated nucleation. Arp2/3 complex initiates a new branched filament by binding to the side of the mother filament and recruiting actin monomers. Components of the Arp2/3 complex remain at the pointed end of the filament (Figure adapted from [11]). The exact mechanism of nucleation remains unclear however it is believed that all seven subunits coordinate to anchor the pointed end of the new filament at a 78° angle to the existing actin network [11]. NPFs act to deliver actin subunits to the Arp2/3 complex at the barbed end, via binding through their WH2 domain – this serves to push the leading edge of a cell forward [12]. It has also been suggested that each component of the complex plays specific roles, with Arp2 and Arp3 interacting with the pointed end of the daughter filament and ARPC2 and ARPC4 binding to the original filament [11]. Figure: Arp2/3-mediated actin polymerization. NPFs (e.g. WASp; Scar) bring together the Arp2/3 complex and actin monomers to nucleate actin filaments that form new branches from the side of pre-existing filaments. The Arp2/3 complex remains at the minus end of the filament. Latest Findings Importantly, the compressive stresses placed upon actin filaments may cause them to bend and curve. Recently this property of the actin cytoskeleton was shown to regulate Arp2/3 mediated nucleation [13]. In this study a significantly higher frequency of filament branches were observed originating from the convex side of curved filaments compared to the concave side. This indicated that nucleation was more likely to occur if an extensional strain existed on the Arp2/3 binding surface on the filament compared to a compressional strain, which generates the concave surface [13].
This bias of Arp2/3 towards branch formation on convex curvature was proposed to represent a mechanosening mechanism whereby detection of filament curvature alters the local cytosekelton in order to reinforce it against the imposing forces [13]. |
References
- Firat-Karalar EN. & Welch MD. New mechanisms and functions of actin nucleation. Curr. Opin. Cell Biol. 2011; 23(1):4-13. [PMID: 21093244]
- Rosenblatt J., Peluso P. & Mitchison TJ. The bulk of unpolymerized actin in Xenopus egg extracts is ATP-bound. Mol. Biol. Cell 1995; 6(2):227-36. [PMID: 7787248]
- Beltzner CC. & Pollard TD. Identification of functionally important residues of Arp2/3 complex by analysis of homology models from diverse species. J. Mol. Biol. 2004; 336(2):551-65. [PMID: 14757065]
- Svitkina TM. & Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 1999; 145(5):1009-26. [PMID: 10352018]
- Korobova F. & Svitkina T. Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol. Biol. Cell 2008; 19(4):1561-74. [PMID: 18256280]
- Schoumacher M., Goldman RD., Louvard D. & Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J. Cell Biol. 2010; 189(3):541-56. [PMID: 20421424]
- Xu J., Wang F., Van Keymeulen A., Herzmark P., Straight A., Kelly K., Takuwa Y., Sugimoto N., Mitchison T. & Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 2003; 114(2):201-14. [PMID: 12887922]
- Suetsugu S., Kurisu S., Oikawa T., Yamazaki D., Oda A. & Takenawa T. Optimization of WAVE2 complex-induced actin polymerization by membrane-bound IRSp53, PIP(3), and Rac. J. Cell Biol. 2006; 173(4):571-85. [PMID: 16702231]
- Lorenz M., Yamaguchi H., Wang Y., Singer RH. & Condeelis J. Imaging sites of N-wasp activity in lamellipodia and invadopodia of carcinoma cells. Curr. Biol. 2004; 14(8):697-703. [PMID: 15084285]
- Stradal TE., Pusch R. & Kliche S. Molecular regulation of cytoskeletal rearrangements during T cell signalling. Results Probl Cell Differ 2006; 43:219-44. [PMID: 17068974]
- Rouiller I., Xu XP., Amann KJ., Egile C., Nickell S., Nicastro D., Li R., Pollard TD., Volkmann N. & Hanein D. The structural basis of actin filament branching by the Arp2/3 complex. J. Cell Biol. 2008; 180(5):887-95. [PMID: 18316411]
- Pollard TD. & Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003; 112(4):453-65. [PMID: 12600310]
- Risca VI., Wang EB., Chaudhuri O., Chia JJ., Geissler PL. & Fletcher DA. Actin filament curvature biases branching direction. Proc. Natl. Acad. Sci. U.S.A. 2012; 109(8):2913-8. [PMID: 22308368]