Read Further…
Cyclic Cdc42/Rac and Rho activation during cell motility is mediated by: Integrin β1 and syndecan-4
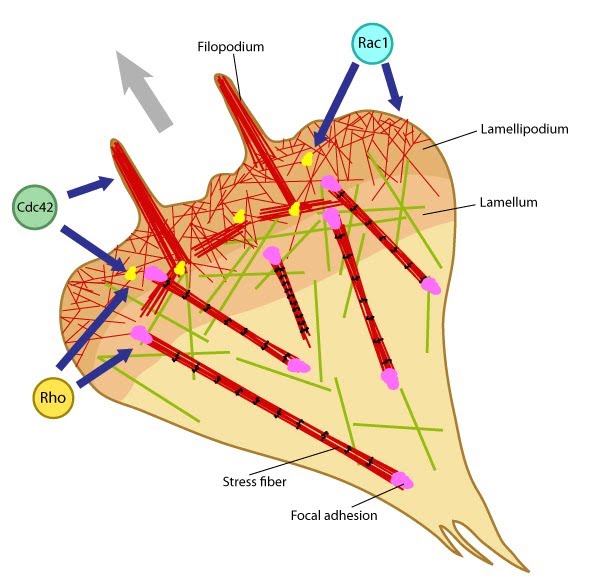
Rac activation and Rho suppression are essential for: Arp2/3 Complex in Actin Branch Nucleation; Focal adhesion initiation and elongation; Lamellipodial translocation
Cdc42 activation and Rho suppression are essential for: Filopodia adherence
Rho activation is essential for: Focal adhesion maturation; Retraction of the trailing edge and Focal adhesion disassembly; Podosome disassembly; Cell polarity
Influenced by: Guidance signals – Attractive cues and Repulsive cues; Activators and Integrin
Rac activation and Rho suppression are essential for: Arp2/3 Complex in Actin Branch Nucleation; Focal adhesion initiation and elongation; Lamellipodial translocation
Cdc42 activation and Rho suppression are essential for: Filopodia adherence
Rho activation is essential for: Focal adhesion maturation; Retraction of the trailing edge and Focal adhesion disassembly; Podosome disassembly; Cell polarity
Influenced by: Guidance signals – Attractive cues and Repulsive cues; Activators and Integrin
The Rho (Ras-homologous) family are Ras-related small GTPases that use the energy from GTP hydrolysis to modulate and control numerous aspects of actin filament dynamics and cytoskeleton structure. Rho GTPases link many cytoplasmic signaling effectors to the actin cytoskeleton [1] (reviewed in [2, 3]) and Rho GTPase activity influences diverse processes such as cell growth, migration, cell shape and cell fate (reviewed in [3, 4, 5]). The specific mechanical and chemical cues that modulate the activity of the more prominent family members, Rho, Rac, and Cdc42, to each of the above mentioned processes is likely to vary between different cell contexts, cell or tissue types, receptors, or stimuli. Although Rho GTPases integrate many signaling events by their interaction with multiple components, these GTPases may act more as permissive factors for cytoskeleton reorganization rather than as direct mediators (reviewed in [6]).
The Rho family members contribute to higher-order actin-based structures such as stress fibers, lamellipodium, dendritic spines, and filopodia [1]. The spatial localization of these GTPases is controlled in different regions of the cell because in certain cases, the activity of one Rho family member antagonizes the activity of another family member (e.g. Rac1 antagonizes RhoA signaling [7]). Conversely, activation of one family member (e.g. Cdc42) can also lead to the stepwise activation of other members (e.g Rac, Rho) [1]. Thus, this significant feedback and crosstalk between the Rho family members complicates the task of constructing one paradigm or linear pathway for Rho-mediated mechanotransduction (reviewed in [8, 9]).