Cellular Structures
Unit 1: Lamellipodia and Lamella
Unit 2: Filopodia Unit 3: Podosomes Unit 4: Invadopodia Unit 5: Cell-Matrix Adhesions Mechanosensors
Cell-cell adhesion molecules
Cell-matrix adhesion receptors
Contribute | Cellular Structures in Mechanosensing and Cell MotilityCells sense their environment through the detection of forces and the measurement of mechanical stimuli, in a process termed mechanosensing. This process is intertwined with cell motility, which in itself facilitates the ability of a cell to sense its surroundings, but also acts as a response to the detection of a stimulus. In this topic we address the complexities of mechanosensing and cell motility by describing the key structures that facilitate these processes (listed below). We explore how these structures carry out their functions and the common machinery that makes this possible.Contents:Unit 1: Lamellipodia and LamellaUnit 2: Filopodia Unit 3: Podosomes Unit 4: Invadopodia Unit 5: Cell-Matrix Adhesions Unit 6: Cell-Cell Adhesions —————- What are the Key Cellular Structures in Mechanosensing and Cell Motility?Cell polarization, changes in cell shape and cell movement can all be thought of as the end result of local mechanotransduction events, orchestrated into a mechanoresponse at the whole cell level. It is however important to note that cells also use spreading and motility as a means of mechanosensing. Such movements mobilize mechanosensors along the cell periphery to measure changes in the external environment.These movements are reliant on the organization of the intracellular architecture (i.e. cytoskeleton), as well as the protrusive and retractile activities of the membrane, along a particular path. This requires the generation of internal forces, but is also modulated by the detection of external forces. Forces that promote cell motility are primarily propagated through the production of actin-based structures (see Figure below) [1] (reviewed in [2, 3]). These higher order structures include lamellipodia, filopodia, invadopodia and podosomes, which are themselves are inherently motile. The motile behavior of lamellipodia and filopodia is dependent on the turnover of cell-matrix and cell-cell adhesions. 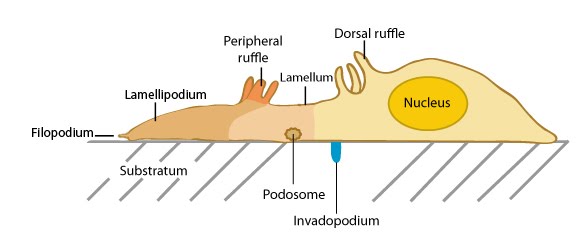 Interactive Figure: Actin-based motility structures. Click on the structure name for more information on its location and function. Actin filaments are a basic structural component found in a number of cellular structures used for cell motility in metazoan cells (insets: filopodium, lamellipodium and lamellum). Certain structures appear sheet-like (e.g. lamellipodia/lamellae, ruffles), whilst others are finger-like (e.g. filopodia, podosomes). In the top panel, the cell is migrating upwards and is attached to a second cell on the right. The bottom panel shows the side view of the migrating cell. Although migrating cells come in many different forms, the leading edge is dominated by the lamellipodium. This structure is highly conserved across cell types and is the key structural element that drives cell migration and spreading. Furthermore, the protein constituents that contribute to lamellipodium formation and maintenance are conserved in many other actin-based extensions (reviewed in [4]). It can therefore be said that the motile behavior of cellular structures, including lamellipodia, filopodia, invadopodia and podosomes, occur through the action of common protein complexes, which are described in this resource as Functional Modules. Additional cytoskeleton components (e.g. microtubules) are important for actin dynamics and for establishing the deposition pattern of the actin network, which both determine the polarity of cell migration (reviewed in [5, 6, 7]). The specific contribution of these components will be discussed throughout. What are the Functional Modules that Mediate Mechanosensing and Cell Motility?In the case of cell motility, each functional module acts to regulate the dynamic state of the cytoskeleton. By viewing cell motility as the sum of all the functional modules that are active at a given time, one can reason that different combinations of active modules will allow the cell to achieve a large number of functions from a limited set of proteins. This results in a variety of different migratory and mechanosensory behaviors.The Functional Modules that mediate motility, either of specific structures or of the entire cell, are:
How are Mechanical Forces Detected?The detection of mechanical force is facilitated through proteins or protein complexes that form mechanosensors. In response to a change in force, mechanosensors commonly exhibit structural rearrangements or geometry-dependent clustering that promote biochemical and mechanotransduction events (reviewed in [8]). These changes in response to force fall into two broad categories:
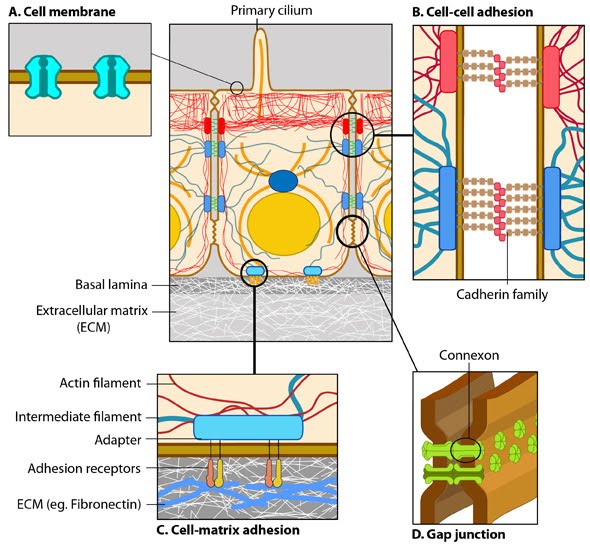
Figure: Mediators of mechanosensing. In the whole cell illustration (middle panel, top row), actin filaments are shown in red, microtubules in yellow and intermediate filaments in blue. Transmembrane and submembrane complexes (solid rectangles in B and C) contain several different components that play a key role in linking the cell exterior to the cell interior. (A) Mechanical deformation of cell membranes can directly modify stress-sensitive transmembrane channels or alter the conformation of mechanosensing proteins/complexes that are linked to the membrane (e.g. caveolae). (B) Numerous cell-cell adhesion molecules (e.g. cadherins) and their associated cytoplasmic anchoring components (e.g. vinculin, actinin) form a continuum between cells and their linked cytoskeleton components, along which forces can be transmitted. Adherens junctions (solid red rectangle) primarily link actin filaments between cells, while desmosomes (solid dark blue rectangle) primarily link the intermediate filaments between cells. (C) Cell-matrix interactions at focal adhesions and hemidesmosomes (solid light blue rectangle) link the actin and intermediate filaments to the underlying matrix, respectively. Cell-matrix linkages, particularly focal adhesions, are a key force sensing unit that greatly influences cell polarity and migration. (D) Mechanical forces can influence the opening of channels within gap junctions to allow communication between cells via the exchange of small water soluble molecules. |
References
- Loisel TP., Boujemaa R., Pantaloni D. & Carlier MF. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 1999; 401(6753):613-6. [PMID: 10524632]
- Carlier MF., Le Clainche C., Wiesner S. & Pantaloni D. Actin-based motility: from molecules to movement. Bioessays 2003; 25(4):336-45. [PMID: 12655641]
- Pollard TD. & Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003; 112(4):453-65. [PMID: 12600310]
- Chhabra ES. & Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat. Cell Biol. 2007; 9(10):1110-21. [PMID: 17909522]
- Goode BL., Drubin DG. & Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr. Opin. Cell Biol. 2000; 12(1):63-71. [PMID: 10679357]
- Fuchs E. & Yang Y. Crossroads on cytoskeletal highways. Cell 1999; 98(5):547-50. [PMID: 10490093]
- Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control? Traffic 2004; 5(7):470-7. [PMID: 15180824]
- Vogel V. & Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006; 7(4):265-75. [PMID: 16607289]
- Schwartz MA. & DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr. Opin. Cell Biol. 2008; 20(5):551-6. [PMID: 18583124]
- Geiger B., Spatz JP. & Bershadsky AD. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009; 10(1):21-33. [PMID: 19197329]
- Bershadsky AD., Balaban NQ. & Geiger B. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003; 19:677-95. [PMID: 14570586]
- Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J. Cell. Sci. 2004; 117(Pt 12):2449-60. [PMID: 15159450]
- Patel A. & Honoré E. Polycystins and renovascular mechanosensory transduction. Nat Rev Nephrol 2010; 6(9):530-8. [PMID: 20625375]