Glossary Term: Myosin ATPase activity: the ‘powerstroke’ cycle
Read Further…
For more detailed information on Myosin read: Essential Info: What are Motor Proteins?; Myosin Isoforms and their Role as Motor Proteins; Functional Module: Myosins and the rearward movement of actin filaments
See also: Contractile bundle; Myosin
See also: Contractile bundle; Myosin
Myosin motor proteins use conformation-dependent changes
associated with nucleotide-binding and hydrolysis to bind to and move
along actin filaments (reviewed in [1, 2, 3]). All myosin proteins bind to
ATP, they have ATPase activity, and they bind to the actin filaments in a
repetitive cycle; the movement of myosin proteins in a particular
direction along the actin filaments is dictated by the structural
orientation of the myosin molecule(s) with relation to the filament.
Conformation-dependent changes associated with ATP-binding and
hydrolysis also alter the affinity of the myosin head for the actin. The
complete cycle of ATP-binding, hydrolysis, and phosphate release is
called the “power stroke” cycle; each step is described in detail below
using myosin II as an example. Each step is also illustrated in the
figure below. A complete round of ATP hydrolysis produces a single
‘step’ or movement of the myosin protein along the filament. Regulation
of actin-activated ATPase activity of myosin by changes in intracellular
free calcium has been known for some time (reviewed in [4]).
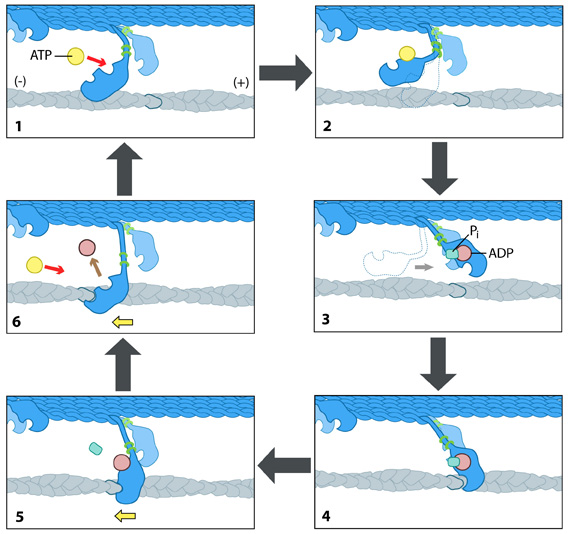
Step 1: At the end of the previous round of movement and the start of the next cycle, the myosin head lacks a bound ATP and it is attached to the actin filament in a very short-lived conformation known as the ‘rigor conformation’.
Step 2: ATP-binding to the myosin head domain induces a small conformational shift in the actin-binding site that reduces its affinity for actin and causes the myosin head to release the actin filament.
Step 3: ATP-binding also causes a large conformational shift in the ‘lever arm’ of myosin that ‘cocks’ the head into a position further along the filament. ATP is then hydrolysed, but the inorganic phosphate and ADP remain bound to myosin.
Step 4: The myosin head makes weak contact with the actin filament and a slight conformational change occurs on myosin that promotes the release of inorganic phosphate.
Step 5: The release of inorganic phosphate reinforces the binding interaction between myosin and actin and subsequently triggers the ‘power stroke’. The power stroke is the key force-generating step used by myosin motor proteins; forces are generated on the actin filament as the myosin protein reverts back to its original conformation.
Step 6: As myosin regains its original conformation, the ADP is released, but the myosin head remains tightly bound to the filament at a new position from where it started, thereby bringing the cycle back to the beginning.
Step 1: At the end of the previous round of movement and the start of the next cycle, the myosin head lacks a bound ATP and it is attached to the actin filament in a very short-lived conformation known as the ‘rigor conformation’.
Step 2: ATP-binding to the myosin head domain induces a small conformational shift in the actin-binding site that reduces its affinity for actin and causes the myosin head to release the actin filament.
Step 3: ATP-binding also causes a large conformational shift in the ‘lever arm’ of myosin that ‘cocks’ the head into a position further along the filament. ATP is then hydrolysed, but the inorganic phosphate and ADP remain bound to myosin.
Step 4: The myosin head makes weak contact with the actin filament and a slight conformational change occurs on myosin that promotes the release of inorganic phosphate.
Step 5: The release of inorganic phosphate reinforces the binding interaction between myosin and actin and subsequently triggers the ‘power stroke’. The power stroke is the key force-generating step used by myosin motor proteins; forces are generated on the actin filament as the myosin protein reverts back to its original conformation.
Step 6: As myosin regains its original conformation, the ADP is released, but the myosin head remains tightly bound to the filament at a new position from where it started, thereby bringing the cycle back to the beginning.
Figure: The “power stroke” mechanism for myosin movement along actin filaments.