Additional Links Essential for: Pulling of Filopodia Function of Lamellipoda and Lamella Retraction of the Trailing Edge Focal adhesion maturation Contribute | Functional Module: Myosins and the Rearward Movement of Actin FilamentsActin filament networks, both within filopodia and lamellipodia, are highly dynamic structures. This characteristic is exemplified in the retrograde motion that is intrinsic to the ‘treadmilling’ mechanism of filament formation. Further to this motion, a number of cellular processes such as filopodial retraction and lamellipodial/lamellal contractions, rely on the rearward movement of the whole filament network or large filament bundles. The retrograde motion of actin treadmilling may play a minor role in aiding these processes, however additional factors are required.One class of proteins that has been implicated in the translocation of F-actin is the myosin motor protein family. It remains unclear which isoforms contribute to this process in specific situations and to what extent [1, 2, 3, 4]. Each member of the myosin family possesses unique structural and functional properties, such as their step size, that determines their ability to engage in F-actin translocation [5]. It has been shown that myosins in general are required for this process to facilitate filopodial retraction [2].
Myosin II has specifically been associated with F-actin retraction in several cell types including neurons [1], fibroblasts [6] and keratocytes [7], with particular emphasis on its role in the lamella and lamellipodia. Initially Myosin II was believed to influence F-actin dynamics and motility from within the lamella, as it had not been observed at the leading edge however it was recently observed within lamellipodia as protrusion reaches its peak, just prior to retraction [8]. This study postulates that myosin II is responsible for the formation and retrograde movement of actin arcs – bundles that form parallel to the leading edge and possibly contact multiple focal adhesions. This movement originates at the lamellipodium and moves rearward into the lamellum, producing a single continuously flowing actin network in the form of arcs [8]. It was consequently proposed that the rate of retrograde movement is reduced as the arcs contact focal adhesions near and within the lamella [8].
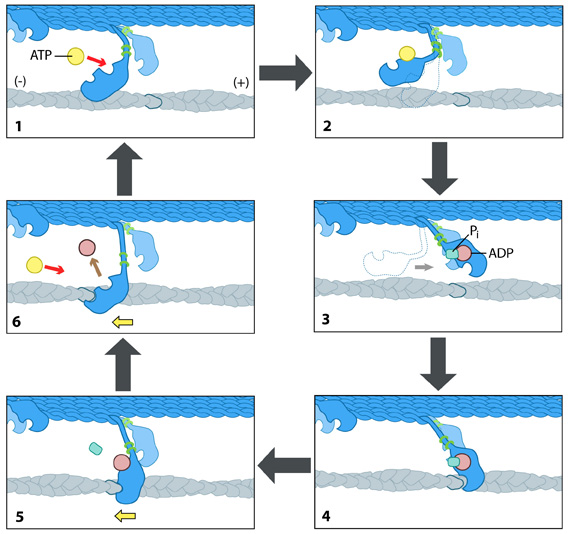
How is F-actin transport carried out? The Powerstroke Mechanism:Each myosin motor protein posseses ATPase activity and functions in a cyclical manner that couples ATP binding and hydrolysis to a conformational change in the protein. This process is known as the ‘powerstroke cycle’ (reviewed in [9, 10, 11]) and is outlined in the steps below using myosin II as an example. This is further described in the Figure below.The direction in which the actin filament will be moved is dictated by the structural orientation of myosin in relation to the filament. A complete round of ATP hydrolysis produces a single ‘step’ or movement of myosin along the actin filament. This process is regulated by changes in the concentration of intracellular free calcium (reviewed in [12]). The steps involved are detailed below: Step 1: At the end of the previous round of movement and the start of the next cycle, the myosin head lacks a bound ATP and it is attached to the actin filament in a very short-lived conformation known as the ‘rigor conformation’. Step 2: ATP binding to the myosin head domain induces a small conformational shift in the actin-binding site that reduces its affinity for actin and causes the myosin head to release the actin filament. Step 3: ATP binding also causes a large conformational shift in the ‘lever arm’ of myosin that bends the myosin head into a position further along the filament. ATP is then hydrolysed, leaving the inorganic phosphate and ADP bound to myosin. Step 4: The myosin head makes weak contact with the actin filament and a slight conformational change occurs on myosin that promotes the release of the inorganic phosphate. Step 5: The release of inorganic phosphate reinforces the binding interaction between myosin and actin and subsequently triggers the ‘power stroke’. The power stroke is the key force-generating step used by myosin motor proteins. Forces are generated on the actin filament as the myosin protein reverts back to its original conformation. Step 6: As myosin regains its original conformation, the ADP is released, but the myosin head remains tightly bound to the filament at a new position from where it started, thereby bringing the cycle back to the beginning. Figure: The “power stroke” mechanism for myosin movement along actin filaments.
Video: The “power stroke” mechanism for myosin movement along actin filaments. [Video uploaded to YouTube by caveman29 and created by www.encognitive.com.] |
References
- Medeiros NA., Burnette DT. & Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol. 2006; 8(3):215-26. [PMID: 16501565]
- Lin CH., Espreafico EM., Mooseker MS. & Forscher P. Myosin drives retrograde F-actin flow in neuronal growth cones. Neuron 1996; 16(4):769-82. [PMID: 8607995]
- Brown J. & Bridgman PC. Role of myosin II in axon outgrowth. J. Histochem. Cytochem. 2003; 51(4):421-8. [PMID: 12642620]
- Diefenbach TJ., Latham VM., Yimlamai D., Liu CA., Herman IM. & Jay DG. Myosin 1c and myosin IIB serve opposing roles in lamellipodial dynamics of the neuronal growth cone. J. Cell Biol. 2002; 158(7):1207-17. [PMID: 12356865]
- Kress H., Stelzer EH., Holzer D., Buss F., Griffiths G. & Rohrbach A. Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity. Proc. Natl. Acad. Sci. U.S.A. 2007; 104(28):11633-8. [PMID: 17620618]
- Verkhovsky AB., Svitkina TM. & Borisy GG. Myosin II filament assemblies in the active lamella of fibroblasts: their morphogenesis and role in the formation of actin filament bundles. J. Cell Biol. 1995; 131(4):989-1002. [PMID: 7490299]
- Svitkina TM., Verkhovsky AB., McQuade KM. & Borisy GG. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J. Cell Biol. 1997; 139(2):397-415. [PMID: 9334344]
- Burnette DT., Manley S., Sengupta P., Sougrat R., Davidson MW., Kachar B. & Lippincott-Schwartz J. A role for actin arcs in the leading-edge advance of migrating cells. Nat. Cell Biol. 2011; 13(4):371-81. [PMID: 21423177]
- Vale RD. & Milligan RA. The way things move: looking under the hood of molecular motor proteins. Science 2000; 288(5463):88-95. [PMID: 10753125]
- Volkmann N. & Hanein D. Actomyosin: law and order in motility. Curr. Opin. Cell Biol. 2000; 12(1):26-34. [PMID: 10679363]
- Hwang W. & Lang MJ. Mechanical design of translocating motor proteins. Cell Biochem. Biophys. 2009; 54(1-3):11-22. [PMID: 19452133]
- Ebashi S. & Endo M. Calcium ion and muscle contraction. Prog. Biophys. Mol. Biol. 1968; 18:123-83. [PMID: 4894870]