The kinesin superfamily of proteins represents a large class of motor proteins that carry cargo along microtubules. The kinesin family comprises at least 45 isoforms in humans alone. These proteins are functionally similar to members of the myosin family such as myosin V or myosin-X, which transports material along actin filaments. Microtubules are generally much longer than actin filaments and as such kinesin may transport cargo over significantly longer distances than the myosins [1].
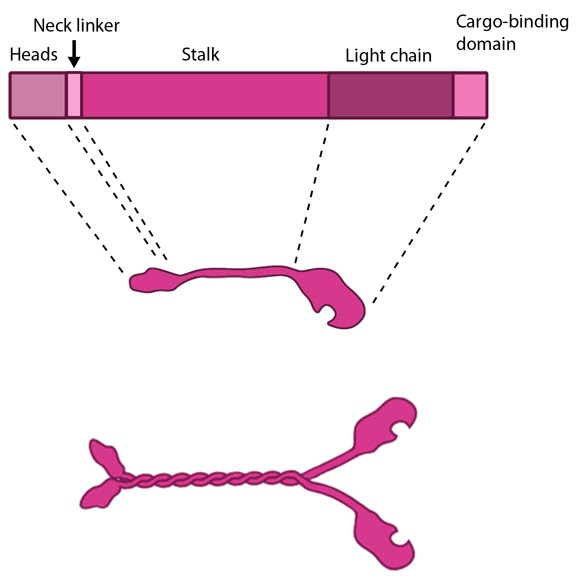
In their inactive state the kinesins exist as monomers, each possessing an N-terminal head (motor region), a neck linker and a globular tail domain. Between the neck linker and tail domain is a long coiled-coil region which serves as a dimerization site that will twist together with the corresponding site of another monomer to produce the active kinesin dimer [1]
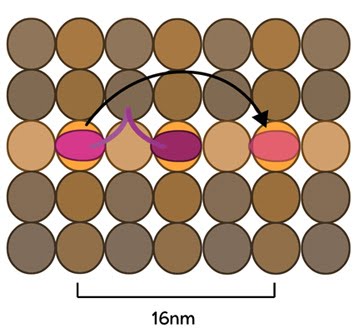
The Kinesin Powerstroke: Conventional kinesins move along microtubule filaments in a manner that resembles human walking. This has been described as an asymmetric ‘hand-over-hand’ mechanism where one head domain steps forward ~16.2nm whilst the other head remains stationary. For each step the head domains take, the cargo is moved 8.1nm along the length of the filament [2, 3] and a single ATP molecule is hydrolyzed. The rate of movement of kinesins is reported to be up to 800nm per second, hydrolyzing approximately 100 ATP molecules per second i.e. 1 ATP molecule per 8.1nm that the cargo is transported [1]. Although microtubules possess up to 13 protofilaments, conventional kinesins walk along single filaments only and avoid binding sites that are theoretically within reach on adjacent protofilaments – binding to these sites would require additional stretching of the neck linker region.
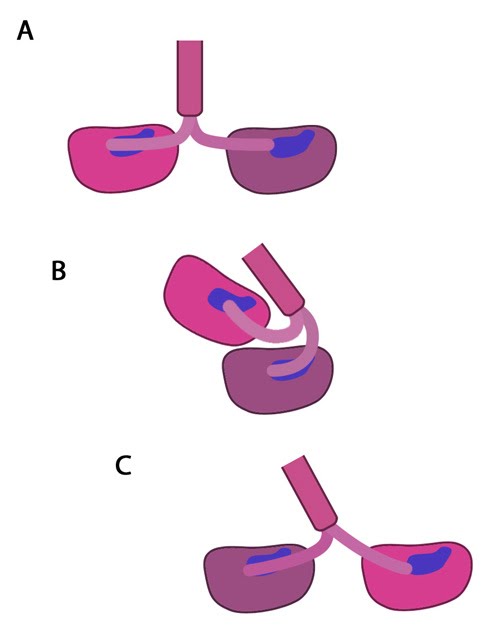
Latest Findings Molecular simulations were recently used to describe the kinetics of the kinesin powerstroke cycle. In this study 3 stages were described that determine step size and direction of the kinesin protein. In the first stage the neck linker undergoes a conformational change upon ATP binding in which it ‘docks’ with the leading head [4, 5, 6, 7]. This essentially involves the alignment and binding of a large portion of the neck linker with particular residues on the leading head. In a starting position where the two heads of kinesin (trailing and leading) are bound to a microtubule, the neck linker that is tethered to the leading head is extended backwards and so does not interact with the docking site on the leading head [8]. Upon commencement of the step process, ‘docking’ occurs which alters the flexibility of the linker region to ensure movement of the trailing head follows along the same line and direction as the leading head [2, 9].
The importance of the neck linker in the kinesin powerstroke has been highlighted by studies that inhibited, removed or replaced this region and reported a range of consequences, from a disruption of the step size to complete inhibition of the walking mechanism [8, 10, 11]. Linker docking could also be said to regulate the second stage of the step in which the trailing head moves forward by ‘tethered diffusion’. In this second stage the trailing head, which is now bound to ADP, will cover the majority of the 16nm long distance to the next binding site. This occurs via anisotropic translational motion (i.e. in a specific direction along the microtubule) and with isotropic rotational motion (i.e. with a uniform rotation). In this stage the trailing head searches for the optimal binding site. During stage 3 interactions between the trailing head and the microtubule binding site occur [2]. Upon binding, ADP diffuses from the nucleotide binding site of the trailing head and the neck linker relaxes away from the docking site. Another ATP molecule will now bind to the leading head and the powerstroke cycle will continue to repeat. Approximately 6pN of force is generated by a single powerstroke of kinesin [1].
Figure: The kinesin powerstroke. In the initial stage of the powerstroke cycle, the neck linker of the leading head is tethered to the head but extended backwards and not interacting with the docking site (A) Following ATP binding and the subsequent conformational change, the neck linker docks with its docking site in the leading head. This pulls the trailing head forward (B). The trailing head continues forward until the neck-linker of the trailing head disengages the docking site. This head finds the next binding site and becomes the leading head (C). The cycle continues, allowing the Kinesin to “walk” down the microtubule filament.