Motor Activity
What are Motor Proteins?[Edit]
Motor proteins, such as myosins and kinesins, can move along cytoskeletal filaments and this force-dependent mechanism is driven by the hydrolysis of ATP molecules (reviewed in [1]). Nucleotide hydrolysis and controlled inorganic phosphate release by motor proteins causes restructuring of core domains that control the association of the motor protein with the filaments, other proteins, and the fresh supply of nucleotides.
Motor proteins propel themselves along the cytoskeleton using a mechanochemical cycle of filament binding, conformational change, filament release, conformation reversal, and filament rebinding. In most cases, the conformational change(s) on the motor protein prevents subsequent nucleotide binding and/or hydrolysis until the prior round of hydrolysis and release is complete.
Controlled hydrolysis of nucleotides and inorganic phosphate release by motor proteins can generate mechanical forces that can be used for:
* Translocating the motor proteins themselves along the filaments
* stabilizing and/or moving the filaments (i.e., contractile stress fibers) & escorting cargo that is attached to the motor protein (e.g., vesicles, organelles, other proteins) to specific regions in the cell
* In most cases, motor proteins transport substances in a particular direction, or polarity, along the filaments. This directionality is achieved by specific conformational changes that allow movement in only one direction.
Controlled hydrolysis of nucleotides and inorganic phosphate release by motor proteins can generate mechanical forces that can be used for:
* Translocating the motor proteins themselves along the filaments
* stabilizing and/or moving the filaments (i.e., contractile stress fibers) & escorting cargo that is attached to the motor protein (e.g., vesicles, organelles, other proteins) to specific regions in the cell
* In most cases, motor proteins transport substances in a particular direction, or polarity, along the filaments. This directionality is achieved by specific conformational changes that allow movement in only one direction.
An Introduction to the Myosin Superfamily of Proteins[Edit]
The most commonly described motor proteins belong to the Myosin superfamily.
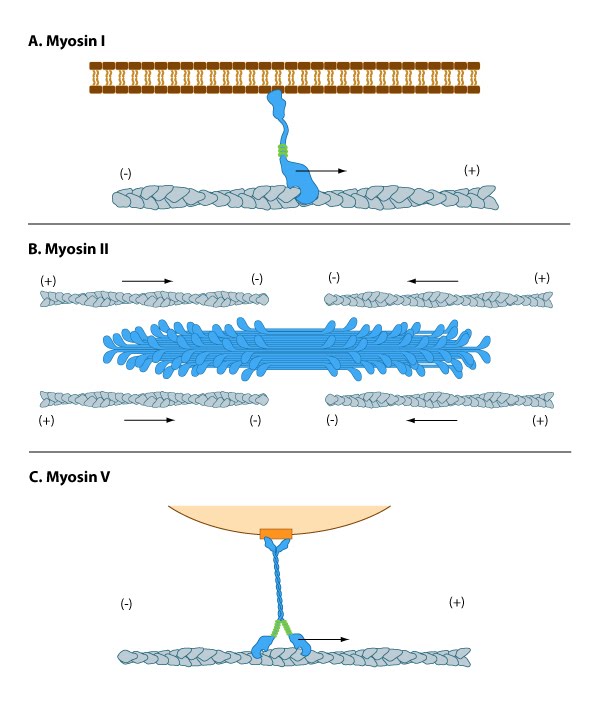
Myosin I has unique tail domain(s) relative to other myosin members which allows myosin I to bind to membrane lipids or to more than one actin filament at a time (see panel ‘A’ in Figure below). Myosin I is primarily involved in intracellular organization, but it also forms a critical component of small cell surface projections in intestinal cells.
Myosin I has unique tail domain(s) relative to other myosin members which allows myosin I to bind to membrane lipids or to more than one actin filament at a time (see panel ‘A’ in Figure below). Myosin I is primarily involved in intracellular organization, but it also forms a critical component of small cell surface projections in intestinal cells.
Myosin II can form higher order assemblies via the extended coiled-coil domains in the heavy chains. For example, the long coiled-coil domains of myosin II interact with the coiled-coiled domains of adjacent myosin II molecules, followed by additional tail-tail interactions with other myosin II assemblies. The resulting myosin II bundle (aka ‘”thick filament”) has several hundred myosin heads oriented in opposite directions at the two ends of the filament. Concerted ATP hydrolysis and movement of the myosin heads along adjacent actin filaments generates a sliding motion that results in shortening or contraction of the interlinked actin filaments (see arrows in panel ‘B’ in Figure below). The action of the actin-myosin system generates forces against the interlinked cytoskeleton network to influence processes such as cell signaling, adhesion, movement, polarity, and cell fate (see “contractile bundle” in the main glossary) [2, 3, 4] (reviewed in [5]). Myosin II is also a critical component of stress fibers and the contractile ring that separates two cells during cell division. For studies which investigate cell contraction and motility, contractile force generated by myosin II can be inhibited using small molecules such as blebbistatin [6] and 2,3-butanedione monoxime (BDM) [7].
Myosin V and Myosin VI In non-muscle cells, actin filaments form an internal track system for cargo transport that is powered by motor proteins such as myosin V and VI ( see panel ‘C’ in Figure below); these myosins use the energy from ATP hydrolysis to transport cargo (such as attached vesicles and organelles) at rates much faster than diffusion. Myosin V contains more light chains and a longer ‘lever arm’ relative to myosin II, which allows myosin V to move in larger steps along the actin filaments (reviewed in [8]).
Myosin V may also colocalize with F-actin bundles. The distribution of myosin V in growth cones is consistent with the role for this myosin in tension production by growth cones. Myosin V may influence the filopodial extension rate by pushing the plasma membrane and creating space for G-actin subunit assembly onto the barbed ends of actin filaments[9, 10]
Myosin VII and Myosin X are important for filopodial assembly and dynamics [11, 12]. Myosin VII is believed to influence the assembly/disassembly of adhesion proteins at the filopodial tip [11] as well as play a role in filopodial extension events. Myosin X activity also influences the filopodia number and overall length with calmodulin-like protein (CLP) modulating this activity by stabilizing myosin X [13]. Myosin X influences transport of materials along filopodial shafts using an ATP-dependent ‘walking’ mechanism. Myosin X binds cell surface receptors, cytoskeleton, Ena/VASP proteins, and membrane phospholipids [11, 14, 15, 16]. Myosin X also has a striking distribution at the tips of filopodia and disrupting its function disrupts filopodium formation [17, 18].
Myosin V and Myosin VI In non-muscle cells, actin filaments form an internal track system for cargo transport that is powered by motor proteins such as myosin V and VI ( see panel ‘C’ in Figure below); these myosins use the energy from ATP hydrolysis to transport cargo (such as attached vesicles and organelles) at rates much faster than diffusion. Myosin V contains more light chains and a longer ‘lever arm’ relative to myosin II, which allows myosin V to move in larger steps along the actin filaments (reviewed in [8]).
Myosin V may also colocalize with F-actin bundles. The distribution of myosin V in growth cones is consistent with the role for this myosin in tension production by growth cones. Myosin V may influence the filopodial extension rate by pushing the plasma membrane and creating space for G-actin subunit assembly onto the barbed ends of actin filaments[9, 10]
Myosin VII and Myosin X are important for filopodial assembly and dynamics [11, 12]. Myosin VII is believed to influence the assembly/disassembly of adhesion proteins at the filopodial tip [11] as well as play a role in filopodial extension events. Myosin X activity also influences the filopodia number and overall length with calmodulin-like protein (CLP) modulating this activity by stabilizing myosin X [13]. Myosin X influences transport of materials along filopodial shafts using an ATP-dependent ‘walking’ mechanism. Myosin X binds cell surface receptors, cytoskeleton, Ena/VASP proteins, and membrane phospholipids [11, 14, 15, 16]. Myosin X also has a striking distribution at the tips of filopodia and disrupting its function disrupts filopodium formation [17, 18].