Clathrin-mediated endocytosis
Content
What is clathrin-mediated endocytosis?[Edit]
Clathrin-mediated endocytosis (CME) is a vesicular transport event that facilitates the internalization and recycling of receptors engaged in a variety of processes, including signal transduction (G-protein and tyrosine kinase receptors), nutrient uptake and synaptic vesicle reformation [1]. Two classical examples of CME are iron-bound transferrin recycling and the uptake of low-density lipoprotein (LDL).
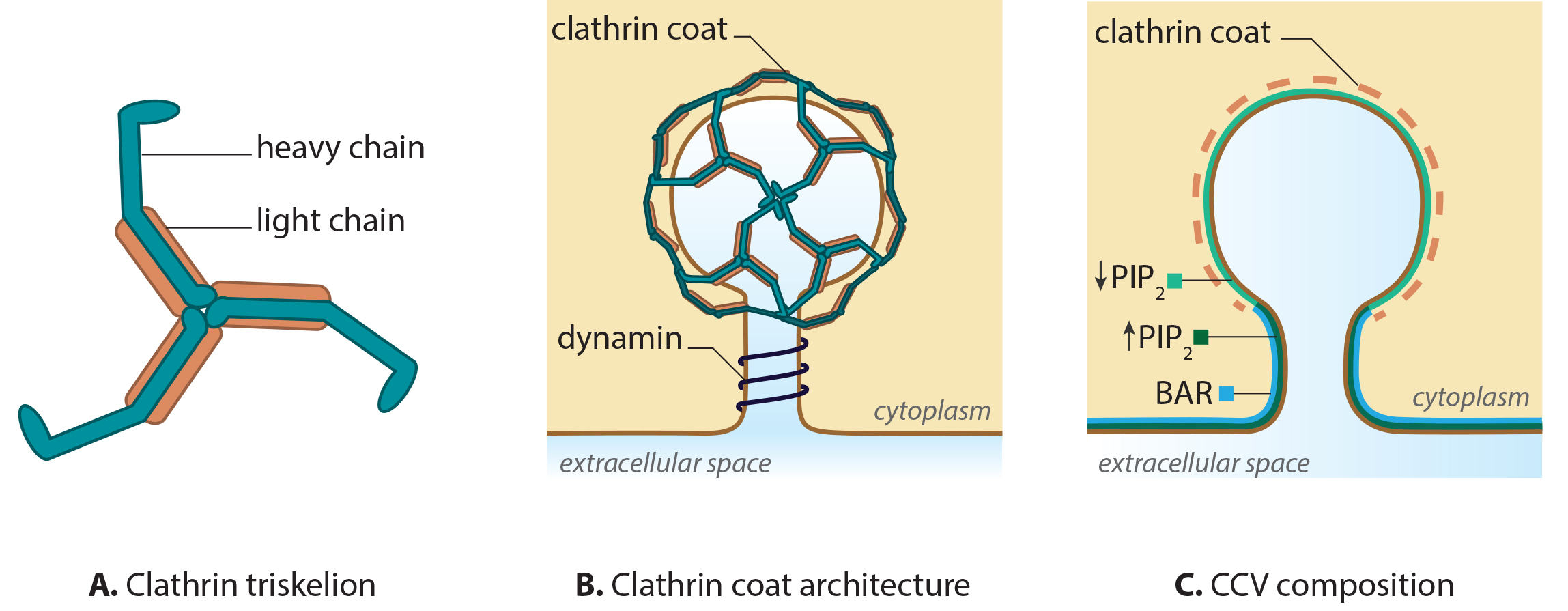
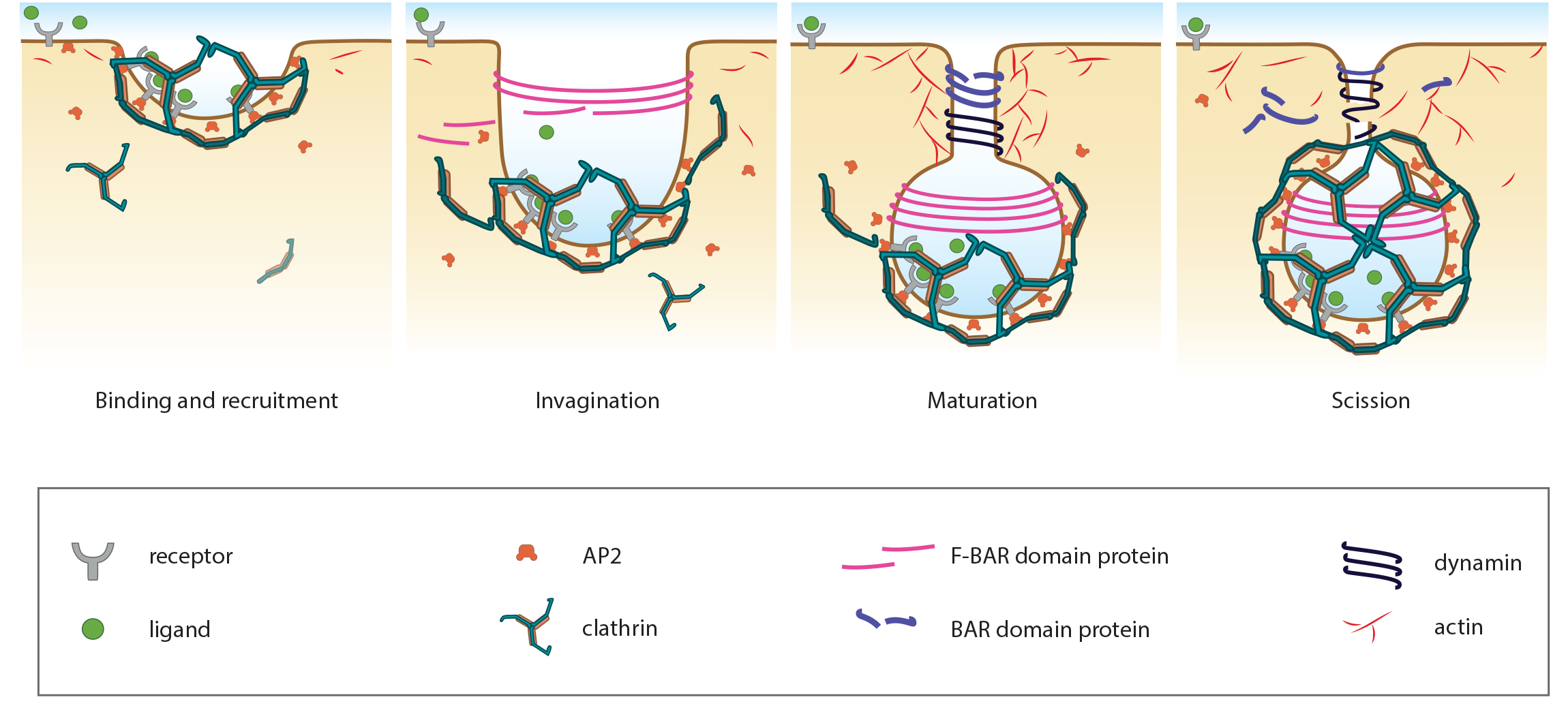
CME is characterized by the involvement of clathrins, which are triskelion-shaped scaffold proteins composed of three heavy and three light chains [2, 3, 4]. Clathrins polymerize around the cytoplasmic face of the invaginated membrane and act as a reinforced mould in which the membrane vesicle may form without direct association with the membrane [5]. Dissociation of the coat occurs rapidly following scission of the vesicle from the membrane.
Initiation of the clathrin complex formation requires the accumulation of phosphatidylinositol‑4,5‑bisphosphate (PIP2) and adaptor proteins, such as AP-2, at the pinching site [6, 7, 8]. In the case of clathrin-coated vesicles (CCV) formed at the trans-Golgi apparatus (TGA), AP-1 is essential [9, 10].
Growth of the clathrin coated pit requires BAR (Bin/Amphiphysin/Rvs) domain proteins [11, 12, 13] and reorganization of the actin network [14]. The final scission step involves BAR domain proteins, dynamin and the dephosphorylation of PIP2. The latter step is suggested to function within a positive feedback loop, with regards to phosphatase activity [15, 16, 17]. The vesicles are then transported and sorted, based on receptor type or membrane composition [18], to the various destinations including the trans-Golgi network, endosomes and vacuoles.
Initiation of the clathrin complex formation requires the accumulation of phosphatidylinositol‑4,5‑bisphosphate (PIP2) and adaptor proteins, such as AP-2, at the pinching site [6, 7, 8]. In the case of clathrin-coated vesicles (CCV) formed at the trans-Golgi apparatus (TGA), AP-1 is essential [9, 10].
Growth of the clathrin coated pit requires BAR (Bin/Amphiphysin/Rvs) domain proteins [11, 12, 13] and reorganization of the actin network [14]. The final scission step involves BAR domain proteins, dynamin and the dephosphorylation of PIP2. The latter step is suggested to function within a positive feedback loop, with regards to phosphatase activity [15, 16, 17]. The vesicles are then transported and sorted, based on receptor type or membrane composition [18], to the various destinations including the trans-Golgi network, endosomes and vacuoles.
Nucleation of Adaptors[Edit]
Clathrin-mediated endocytosis is triggered by phosphatidylinositol-4,5-bisphosphate (PIP2) accumulation within the plasma membrane. PIP2 accumulates as a result of phosphoinositide catalysis by the lipid kinases, phosphatidylinositol-4-kinase (PI4K) and phosphatidylinositol-4-phosphate-5-kinase (PI4P5K) [19, 7] and hydrolysis by phosphatases. The adaptor proteins (AP-2 in mammals or Sla1 in budding yeast) collapse on PIP2 in a stochastic manner and form an adaptor-PIP2 complex. The complex is stabilized by accessory proteins, such as clathrin-associated sorting proteins (CLASPs) [20], actin and myosin I [21, 22, 23]. AP-2 is autoinhibited in the cytosol when the levels of PIP2 or cargo are low. This prevents inappropriate clathrin recruitment. Binding of PIP2 and cargo to AP-2, which results from PIP2 accumulation, activates AP-2, allowing the complex to bind clathrin. The presence of cargo further stabilizes the nucleation process [24]. The mechanism by which certain sites are chosen for endocytosis is not clear. In budding yeasts, large immobile assemblies of proteins on the plasma membrane called eisosomes have been identified. Eisosomes mark the sites of endocytosis, and are primarily comprised of two proteins, Pil1 and Lsp1 [25].
Formation of the Clathrin Coated Pit[Edit]
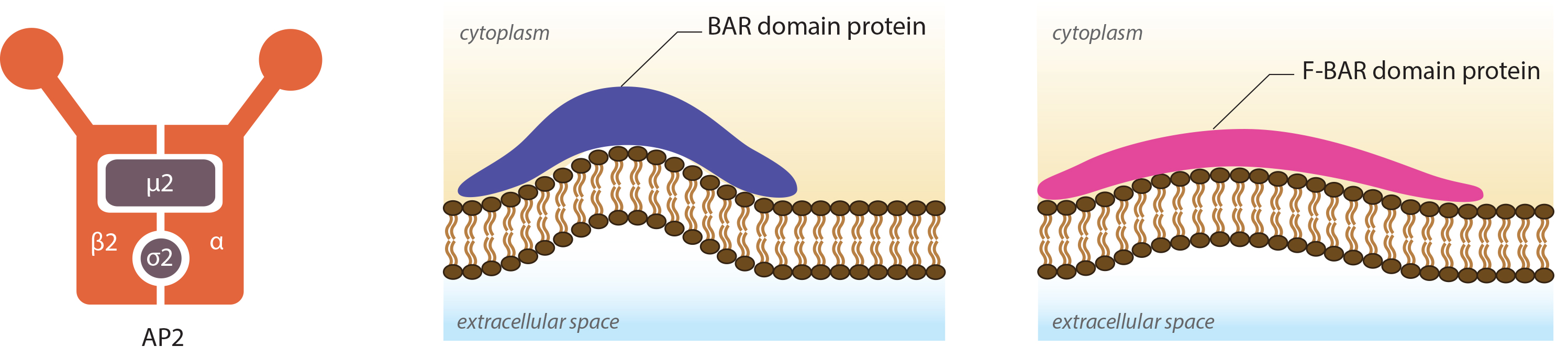
Adaptor proteins such as AP-2, AP180 and CALM (Clathrin-assembly lymphoid myeloid leukaemia protein), which accumulate within the lipid bilayer, are responsible for the recruitment of the triskelion shaped Clathrin trimer. This trimer does not interact with the membrane directly but instead forms a reinforcing lattice structure that acts as a mold in which membrane vesicles may develop. Its influence on membrane curvature is via the adapter proteins which are anchored to the lipid bilayer. Importantly, the adaptor proteins also participate directly in membrane bending and vesicle size determination [26].
The formation of clathrin-coated pits (CCPs) requires various actin-binding proteins such as those belonging to the BAR (Bin/Amphiphysin/Rvs) superfamily. These include amphiphysin and endophilin in mammals and Rvs161p and Rvs167p in yeast [27]. The role of these BAR proteins is in membrane deformation, essentially promoting its tubulation. By binding to negatively charged membranes, a positive curvature is obtained that follows the concave topology of the protein’s amphipathic α -helix dimer [28, 29]. F-BAR proteins, which belong to a sub-family of the BAR superfamily, possess a larger domain that is also concave in shape, yet shallower in its curvature. These proteins are proposed to generate vesicles with a larger radius compared to those proteins with possessing a BAR domain [30, 31]. In both cases the proteins may act as curvature sensors that reform the membrane into a shape to which they can readily bind [32]. In the case of clathrin mediated endocytosis, the F-BAR proteins are believed to arrive at the site of clathrin-coated pit formation, before the BAR proteins, and as such may also be involved in nucleation of the CCP [33].
While the BAR domain proteins facilitate tubulation of the membrane, the adaptor proteins including AP-2 or those that possess the epsin N-terminal homology (ENTH) domain such as epsin [34], or the AP-180 N-terminal homology (ANTH) domains such as AP-180 continue to recruit the clathrin triskelion and other regulatory proteins required for the later stages of clathrin-coated vesicle (CCV) formation. Both the ENTH and ANTH domains are highly homologous and bind inositol phospholipids; especially PIP2 [35]. Although both protein subclasses stimulate the formation of a clathrin triskelia network, only proteins possessing the ENTH domain influence membrane curvature, with the clathrin lattice produced by AP-180 stimulation having been shown to remain flat [36]. This influence from the ENTH domains is believed to result from the formation of an additional α –helix ‘α0’ between the ENTH domain and the PIP molecule [36]. It has been proposed that insertion of this domain between the lipid heads of the membrane bilayer may be sufficient to alter membrane curvature alone; however it may also be a synergistic response with clathrin assembly [35].
The formation of clathrin-coated pits (CCPs) requires various actin-binding proteins such as those belonging to the BAR (Bin/Amphiphysin/Rvs) superfamily. These include amphiphysin and endophilin in mammals and Rvs161p and Rvs167p in yeast [27]. The role of these BAR proteins is in membrane deformation, essentially promoting its tubulation. By binding to negatively charged membranes, a positive curvature is obtained that follows the concave topology of the protein’s amphipathic α -helix dimer [28, 29]. F-BAR proteins, which belong to a sub-family of the BAR superfamily, possess a larger domain that is also concave in shape, yet shallower in its curvature. These proteins are proposed to generate vesicles with a larger radius compared to those proteins with possessing a BAR domain [30, 31]. In both cases the proteins may act as curvature sensors that reform the membrane into a shape to which they can readily bind [32]. In the case of clathrin mediated endocytosis, the F-BAR proteins are believed to arrive at the site of clathrin-coated pit formation, before the BAR proteins, and as such may also be involved in nucleation of the CCP [33].
While the BAR domain proteins facilitate tubulation of the membrane, the adaptor proteins including AP-2 or those that possess the epsin N-terminal homology (ENTH) domain such as epsin [34], or the AP-180 N-terminal homology (ANTH) domains such as AP-180 continue to recruit the clathrin triskelion and other regulatory proteins required for the later stages of clathrin-coated vesicle (CCV) formation. Both the ENTH and ANTH domains are highly homologous and bind inositol phospholipids; especially PIP2 [35]. Although both protein subclasses stimulate the formation of a clathrin triskelia network, only proteins possessing the ENTH domain influence membrane curvature, with the clathrin lattice produced by AP-180 stimulation having been shown to remain flat [36]. This influence from the ENTH domains is believed to result from the formation of an additional α –helix ‘α0’ between the ENTH domain and the PIP molecule [36]. It has been proposed that insertion of this domain between the lipid heads of the membrane bilayer may be sufficient to alter membrane curvature alone; however it may also be a synergistic response with clathrin assembly [35].
Invagination and Maturation of the clathrin-coated vesicle[Edit]
Clathrin-coated vesicle maturation incorporates the activities of a range of proteins. Actin, myosin and WASP all have important roles in the formation and stabilization of clathrin-coated pits (CCPs) [37, 38, 39]. Evidence also suggests that F-actin influences the lateral movement of CCPs, which effects the subsequent growth of the vesicle [37]. Some proteins act directly on the membrane or clathrin coat whilst others contribute indirectly through regulatory roles or via the adaptor proteins. One protein with a major role in CCP maturation is dynamin, a GTPase that also facilitates scission of the vesicle from the plasma membrane [40]. Although there is no clear involvement of dynamin in yeast, the yeast dynamin-like protein Vps1 has been implicated in endocytosis in this model organism [41].
Dynamin is a non-classical GTPase, with a low affinity to GTP, but a high rate of GTP hydrolysis and GDP dissociation [42]. It is suggested to detect the progression of invagination and therefore act as a sensor for the maturation of the CCP to a CCV [40]. This is facilitated by its unique, non-classical GTPase properties, which best fit the function of dynamin to a two-step process of invagination: 1) the pre-collar rate limiting stage and 2) the post-collar fast assembly stage stimulated by GTP hydrolysis [43]. The second stage is crucial for the scission of clathrin-coated vesicles. It has been proposed that dynamin controls the progress of CCV through a checkpoint system which may either lead to CCV maturation or abortion [40]. To initiate abortion, dynamin will bind auxilin and Hsc70, which will in turn begin disassembly of the clathrin coat, a function normally carried out after scission of the mature vesicle [44]. Similarly, progression through to maturation is governed by dynamin’s recruitment of various accessory proteins that monitor and control membrane curvature (amphiphysin/endophilin) or cargo (SNX9, grb2, TTP) [40].
As mentioned, various nucleation promotion factors including N-WASP, Arp2/3 and cortactin are also recruited to the endocytic site and promote actin polymerization during CCP maturation. These have been shown to co-localize with clathrin at the endocytic site [45, 46, 47] and are indicative of a role for actin cytoskeleton dyamics in clathrin-coated vesicle formation. Although this is well established in yeast cells [48, 49], the influence of the actin cytoskeleton on CCV maturation in mammalian cells is controversial.
In support of a correlation between actin cytoskeleton dynamics and CCV maturation, cortactin was shown to bind to dynamin-2 through its Src-homology 3 (SH3) domain [47, 50] and it was also reported that actin arrives at the endocytic site following a burst of dynamin recruitment [51]. As dynamin is primarily active in the later stages of CCV maturation, just prior to and during scission, it was proposed that actin’s influence is particularly prominent in the later stages of clathrin-mediated endocytosis [51, 49].
Other studies however suggest the influence of actin cytoskeleton dynamics lies in scission of the vesicle, and not maturation or formation of the CCV. This was suggested when vesicle scission was reported to coincide with the peak level of Arp2/3 mediated actin polymerization [39] and when an 82% reduction in CCV scission was reported following latrunculin B treatment of Swiss 3T3 cells [39]. Although This latter study failed to assess CCV formation, their results were supported by latrunculin A and jasplakinalide treatment of the same cell line. Here, an increase in the number of invaginated CCPs was detected indicating the drugs only inhibited CME at the scission stage [37].
It may be the case that an intact cytoskeleton at the endocytic site is not mandatory and its contribution to CCV formation and maturation will depend on the cell type and environment, as was suggested by a study that considered the importance of an intact actin cytoskeleton by inducing its depolymerization, or arresting its polymerization, whilst monitoring the process of clathrin-mediated endocytosis. [40]. Alternative roles for the actin cytoskeleton were proposed when an increase in tubule growth was observed in COS-7 cells following treatment with Latrunculin B. It was concluded here that under normal circumstances, the actin cytoskeleton, along with dynamin, regulate tubule growth by maintaining membrane rigidity [52].
Dynamin is a non-classical GTPase, with a low affinity to GTP, but a high rate of GTP hydrolysis and GDP dissociation [42]. It is suggested to detect the progression of invagination and therefore act as a sensor for the maturation of the CCP to a CCV [40]. This is facilitated by its unique, non-classical GTPase properties, which best fit the function of dynamin to a two-step process of invagination: 1) the pre-collar rate limiting stage and 2) the post-collar fast assembly stage stimulated by GTP hydrolysis [43]. The second stage is crucial for the scission of clathrin-coated vesicles. It has been proposed that dynamin controls the progress of CCV through a checkpoint system which may either lead to CCV maturation or abortion [40]. To initiate abortion, dynamin will bind auxilin and Hsc70, which will in turn begin disassembly of the clathrin coat, a function normally carried out after scission of the mature vesicle [44]. Similarly, progression through to maturation is governed by dynamin’s recruitment of various accessory proteins that monitor and control membrane curvature (amphiphysin/endophilin) or cargo (SNX9, grb2, TTP) [40].
As mentioned, various nucleation promotion factors including N-WASP, Arp2/3 and cortactin are also recruited to the endocytic site and promote actin polymerization during CCP maturation. These have been shown to co-localize with clathrin at the endocytic site [45, 46, 47] and are indicative of a role for actin cytoskeleton dyamics in clathrin-coated vesicle formation. Although this is well established in yeast cells [48, 49], the influence of the actin cytoskeleton on CCV maturation in mammalian cells is controversial.
In support of a correlation between actin cytoskeleton dynamics and CCV maturation, cortactin was shown to bind to dynamin-2 through its Src-homology 3 (SH3) domain [47, 50] and it was also reported that actin arrives at the endocytic site following a burst of dynamin recruitment [51]. As dynamin is primarily active in the later stages of CCV maturation, just prior to and during scission, it was proposed that actin’s influence is particularly prominent in the later stages of clathrin-mediated endocytosis [51, 49].
Other studies however suggest the influence of actin cytoskeleton dynamics lies in scission of the vesicle, and not maturation or formation of the CCV. This was suggested when vesicle scission was reported to coincide with the peak level of Arp2/3 mediated actin polymerization [39] and when an 82% reduction in CCV scission was reported following latrunculin B treatment of Swiss 3T3 cells [39]. Although This latter study failed to assess CCV formation, their results were supported by latrunculin A and jasplakinalide treatment of the same cell line. Here, an increase in the number of invaginated CCPs was detected indicating the drugs only inhibited CME at the scission stage [37].
It may be the case that an intact cytoskeleton at the endocytic site is not mandatory and its contribution to CCV formation and maturation will depend on the cell type and environment, as was suggested by a study that considered the importance of an intact actin cytoskeleton by inducing its depolymerization, or arresting its polymerization, whilst monitoring the process of clathrin-mediated endocytosis. [40]. Alternative roles for the actin cytoskeleton were proposed when an increase in tubule growth was observed in COS-7 cells following treatment with Latrunculin B. It was concluded here that under normal circumstances, the actin cytoskeleton, along with dynamin, regulate tubule growth by maintaining membrane rigidity [52].
Narrowing of the neck[Edit]
In the final stages of clathrin-coated vesicle (CCV) formation, Phosphatidylinositol-4,5-bisphosphate (PIP2) undergoes a dephosphorylation by phosphatases such as synaptojanin 1 (Synj1) [53]. Not only does this inhibit PIP2’s signal transduction capabilities and ensures that the adaptor proteins selectively accumulate within the plasma membrane, but it also promotes further membrane curvature at the vesicle bud, dissociation of the BAR domain proteins (BDPs) and closing of the membrane bud neck [53].
Synj1 has been shown to be recruited by endophilin 1 [53, 54], a BDP that arrives at the endocytic site later than other adaptor proteins [55]. Through its partnership with endophilin, which possesses a curve-sensing role, Synj1 may preferentially hydrolyze PIP2 in highly curved membranes [56].
Normally PIP2 is protected from hydrolysis by the BDPs. With increasing membrane curvature however, the binding strength of the BDPs is weakened, exposing more membrane (and hence PIP2) to the phosphatases. This positive feedback loop promotes continued dephosphorylation of PIP2, and leads to further increases in membrane curvature and subsequently a narrowing of the endocytic bud neck [53].
Current evidence suggests that membrane curvature enhances phosphatase function by providing greater access to the lipid head groups for both increased binding and hydrolysis activity [32]. This is indeed the case with phospholipase-c activity [57], and it has also been confirmed using cell cell-free assays with liposomes of varying sizes that Synj1 possesses an intrinsic preference for smaller vesicles with highly curved membranes [56].
Normally PIP2 is protected from hydrolysis by the BDPs. With increasing membrane curvature however, the binding strength of the BDPs is weakened, exposing more membrane (and hence PIP2) to the phosphatases. This positive feedback loop promotes continued dephosphorylation of PIP2, and leads to further increases in membrane curvature and subsequently a narrowing of the endocytic bud neck [53].
Current evidence suggests that membrane curvature enhances phosphatase function by providing greater access to the lipid head groups for both increased binding and hydrolysis activity [32]. This is indeed the case with phospholipase-c activity [57], and it has also been confirmed using cell cell-free assays with liposomes of varying sizes that Synj1 possesses an intrinsic preference for smaller vesicles with highly curved membranes [56].
Scission of clathrin-coated vesicles[Edit]
As mentioned, in mammalian CME the GTPase dynamin is believed to play important roles in invagination and clathrin-coated pit (CCP) maturation [58]. Its binding partners endophilin and amphiphysin, can also induce tubulation of the vesicles and have been shown to copolymerize with dynamin [59]. It has been suggested that constriction of the rings may be sufficient to pinch off the CCVs from the plasma membrane [60], however, it is generally accepted that additional factors or forces will contribute to promote scission. In support of this is evidence indicating that in the presence of GTP, dynamin will undergo a twisting action and subsequently apply a longitudinal tension on the tubule that results in its breakage [61]. A similar observation was made during experiments using lipid nanotubes containing PIP2. In this case dynamin was observed to form a molecular spring that underwent conformational changes (from compressed to relaxed) in the presence of GTP. It was proposed in this study that vesicle scission would result from dynamin pulling the membrane inward and finally breaking the tubule [62]. In shorter tubules or bud necks however, it has been suggested that additional factors may be required to promote vesicle scission [59].
Scission and dynamin accumulation is known to correspond to an abrupt decrease in PIP2 levels, and this has been attributed to the recruitment of Synj1 by the BAR domain proteins [53]. Mathematical modeling demonstrates that the site of scission will be at the phase boundary where the amount of PIP2 decreases sharply [63]. It has been postulated that this depletion of PIP2 results in a weakened hydrogen bond network in the upper part of the budding membrane whilst a strong hydrogen bond network remains in the lower part of the budding membrane. A subsequent imbalance in surface tension results [32]. Also impacting membrane tension, and possibly contributing to vesicle scission, is the actin cytoskeleton. It has been shown that polymerization of the actin cytoskeleton is not crucial for vesicle scission in all cells; however, a high level of co-ordination was reported.
Scission and dynamin accumulation is known to correspond to an abrupt decrease in PIP2 levels, and this has been attributed to the recruitment of Synj1 by the BAR domain proteins [53]. Mathematical modeling demonstrates that the site of scission will be at the phase boundary where the amount of PIP2 decreases sharply [63]. It has been postulated that this depletion of PIP2 results in a weakened hydrogen bond network in the upper part of the budding membrane whilst a strong hydrogen bond network remains in the lower part of the budding membrane. A subsequent imbalance in surface tension results [32]. Also impacting membrane tension, and possibly contributing to vesicle scission, is the actin cytoskeleton. It has been shown that polymerization of the actin cytoskeleton is not crucial for vesicle scission in all cells; however, a high level of co-ordination was reported.
Uncoating of clathrin-coated vesicles[Edit]
Uncoating is the process by which clathrin is removed from clathrin-coated vesicles (CCVs). In mammals, this ATP dependent process is driven by the 70kDa molecular chaperone ‘Heat shock cognate protein’ (Hsc70) [64]. Two members of the J domain family of proteins, namely auxilin, which is expressed in neurons [65], and cyclin-G dependent kinase (GAK), which is expressed ubiquitously but enriched in the Golgi [66, 67], are also crucial to the mechanism. These proteins act as co-chaperones to Hsc70. Both auxilin and GAK have been shown to bind to the clathrin trimer itself (via its heavy chain) and the adaptor protein AP-2. It is through these associations that they can repeatedly recruit Hsc70 to the endocytic site [68]. Binding of Auxilin or GAK to Hsc70 occurs via the histidine-proline-aspartic acid motif of the J domain [66]
Although the exact mechanics of clathrin disassembly remain unclear, it has been established that for every clathrin triskelion removed from the vesicle coat, a single auxilin molecule is required. Although studies have shown that more auxilin can bind to the clathrin coat [68, 69], it is clear that only one is required for optimal disassembly [70]. In contrast, three Hsc70 proteins are required to carry out the disassembly of a single clathrin triskelion and it has been shown in vitro that reducing the concentration of Hsc70:ATP stalls the reaction before its completion. Addition of more Hsc70:ATP allows disassembly to resume [70]. Each of the three Hsc70 proteins is powered by the hydrolysis of ATP and therefore three ATP molecules are required for the disassembly of a single clathrin triskelion from the cage [71, 72].
In vitro biochemical studies suggest that the uncoating reaction is sensitive to pH, with efficient recruitment and binding of the chaperones occurring at pH6.0 and Hsc70 mediated dissocation of the clathrin triskelion occurring at pH7.0. At pH6.0 it was found that despite the ATPase activity of Hsc70 continuing, it was unable to disassemble the clathrin coat [73]
The rate of disassembly ranges substantially depending on the parameters of the experiments performed in its assessment. Measured in half-life (t½), studies using centrifugation-based experiments, which incorporate the adaptor proteins, report a slow disassembly of between 2 to 10 minutes [72, 74, 75]. Experiments that measure light scattering report much faster disassembly rates of approximately 10 seconds [70]. These differences have been attributed to the stabilization each adaptor protein confers to the clathrin cage [70]. Furthermore, additional in vitro experiments have shown that the time course of uncoating is non-linear and biphasic. Phase one involves a rapid burst of uncoating, followed by a second, slower phase of steady-state uncoating [76, 71]. A low ATP hydolysis rate is observed when ATP is bound in complex with Hsc70 and this has been suggested to govern the rate of uncoating following the initial burst of activity in phase one which subsequently limits the amount of disassembly that can follow [71].
Following dissociation of the clathrin triskelion from the clathrin cage, hsc70 remains bound to these clathrin triskelions, preventing their improper polymerization in the cytoplasm [76].
Although the exact mechanics of clathrin disassembly remain unclear, it has been established that for every clathrin triskelion removed from the vesicle coat, a single auxilin molecule is required. Although studies have shown that more auxilin can bind to the clathrin coat [68, 69], it is clear that only one is required for optimal disassembly [70]. In contrast, three Hsc70 proteins are required to carry out the disassembly of a single clathrin triskelion and it has been shown in vitro that reducing the concentration of Hsc70:ATP stalls the reaction before its completion. Addition of more Hsc70:ATP allows disassembly to resume [70]. Each of the three Hsc70 proteins is powered by the hydrolysis of ATP and therefore three ATP molecules are required for the disassembly of a single clathrin triskelion from the cage [71, 72].
In vitro biochemical studies suggest that the uncoating reaction is sensitive to pH, with efficient recruitment and binding of the chaperones occurring at pH6.0 and Hsc70 mediated dissocation of the clathrin triskelion occurring at pH7.0. At pH6.0 it was found that despite the ATPase activity of Hsc70 continuing, it was unable to disassemble the clathrin coat [73]
The rate of disassembly ranges substantially depending on the parameters of the experiments performed in its assessment. Measured in half-life (t½), studies using centrifugation-based experiments, which incorporate the adaptor proteins, report a slow disassembly of between 2 to 10 minutes [72, 74, 75]. Experiments that measure light scattering report much faster disassembly rates of approximately 10 seconds [70]. These differences have been attributed to the stabilization each adaptor protein confers to the clathrin cage [70]. Furthermore, additional in vitro experiments have shown that the time course of uncoating is non-linear and biphasic. Phase one involves a rapid burst of uncoating, followed by a second, slower phase of steady-state uncoating [76, 71]. A low ATP hydolysis rate is observed when ATP is bound in complex with Hsc70 and this has been suggested to govern the rate of uncoating following the initial burst of activity in phase one which subsequently limits the amount of disassembly that can follow [71].
Following dissociation of the clathrin triskelion from the clathrin cage, hsc70 remains bound to these clathrin triskelions, preventing their improper polymerization in the cytoplasm [76].
Transport and Fusion to Early Endosomes[Edit]
Different cytoskeletal networks have been implicated in the transport of clathrin-coated vesicles (CCVs). In yeast, the actin cytoskeleton traffics dissociated CCVs [77], whilst in mammals the microtubule network transports is involved in the sorting of CCVs to distinct populations of early endosomes [78]. Two early endosomal populations, static and dynamic, have been identified based on their motility within the cell. CCVs that rapidly engage the microtubule network after detachment from the plasma membrane are more likely to be sorted to dynamic early endosomes [78]. This sorting process involves the use of different adaptors [26], for example; CCVs marked with the adaptor protein AP-2 generally sort to the static population of early endosomes [78].