Lamellipodium
Content
What are the lamellipodia and lamella?[Edit]
The lamellipodia and lamella are plate-like extensions of the cell that play crucial roles in both cell motility and migration, and mechanosensing. These structures form and function over distinct steps.
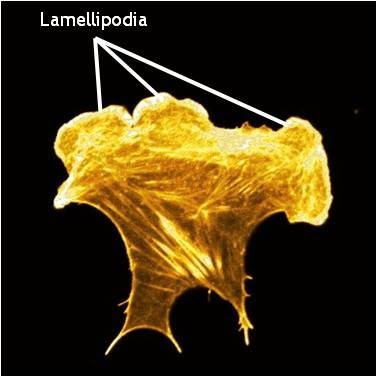 Figure 1. Lamellipodia in a cell stained for F-actin: A mouse embryonic fibroblast of the RPTPa cell line, plated on a fibronectin coated glass cover slip. The cell was transfected with RFP-Lifeact (a kind gift from Dr Roland Wedlich-Soldner, Max Planck Institute of Biochemistry, Germany), which labels F-actin in living cells. The cell was imaged using a Nikon A1Rsi confocal microscope at 60x magnification and false coloured yellow. [Image captured by Wei Wei Luo, Mechanobiology Institute, Singapore]
Lamellipodia are thin, sheet-like membrane protrusions found at the leading edge (front) of motile cells such as endothelial cells, neurons, immune cells and epithelial cells. These structures are generally devoid of major organelles and are instead composed of a dense and dynamic network of actin filaments. The forces generated by actin filament assembly at the leading edge induce membrane protrusion and subsequent lamellipodial growth. This has been extensively reviewed in [1, 2, 3, 4,5].
Figure 1. Lamellipodia in a cell stained for F-actin: A mouse embryonic fibroblast of the RPTPa cell line, plated on a fibronectin coated glass cover slip. The cell was transfected with RFP-Lifeact (a kind gift from Dr Roland Wedlich-Soldner, Max Planck Institute of Biochemistry, Germany), which labels F-actin in living cells. The cell was imaged using a Nikon A1Rsi confocal microscope at 60x magnification and false coloured yellow. [Image captured by Wei Wei Luo, Mechanobiology Institute, Singapore]
Lamellipodia are thin, sheet-like membrane protrusions found at the leading edge (front) of motile cells such as endothelial cells, neurons, immune cells and epithelial cells. These structures are generally devoid of major organelles and are instead composed of a dense and dynamic network of actin filaments. The forces generated by actin filament assembly at the leading edge induce membrane protrusion and subsequent lamellipodial growth. This has been extensively reviewed in [1, 2, 3, 4,5].
The lamellum or lamella (LM; plural lamellae) localizes behind the lamellipodium and is usually the broadest structure in motile cells (typically 10-15 µm wide). Consisting primarily of condensed linear actin bundles, the actin filament network of the lamella is more stable and less dynamic than that of lamellipodia [6] and may also resist compression. Lamellae feature stronger and more mature adhesion sites (reviewed in [7]) and also contain myosin II, a motor protein that is essential in cell motility.
Basic Description
 Figure 1. Lamellipodia in a cell stained for F-actin: A mouse embryonic fibroblast of the RPTPa cell line, plated on a fibronectin coated glass cover slip. The cell was transfected with RFP-Lifeact (a kind gift from Dr Roland Wedlich-Soldner, Max Planck Institute of Biochemistry, Germany), which labels F-actin in living cells. The cell was imaged using a Nikon A1Rsi confocal microscope at 60x magnification and false coloured yellow. [Image captured by Wei Wei Luo, Mechanobiology Institute, Singapore]
Figure 1. Lamellipodia in a cell stained for F-actin: A mouse embryonic fibroblast of the RPTPa cell line, plated on a fibronectin coated glass cover slip. The cell was transfected with RFP-Lifeact (a kind gift from Dr Roland Wedlich-Soldner, Max Planck Institute of Biochemistry, Germany), which labels F-actin in living cells. The cell was imaged using a Nikon A1Rsi confocal microscope at 60x magnification and false coloured yellow. [Image captured by Wei Wei Luo, Mechanobiology Institute, Singapore]The lamellum or lamella (LM; plural lamellae) localizes behind the lamellipodium and is usually the broadest structure in motile cells (typically 10-15 µm wide). Consisting primarily of condensed linear actin bundles, the actin filament network of the lamella is more stable and less dynamic than that of lamellipodia [6] and may also resist compression. Lamellae feature stronger and more mature adhesion sites (reviewed in [7]) and also contain myosin II, a motor protein that is essential in cell motility.
Role of the Lamellipodia in Mechanosensing and Cell Motility[Edit]
During cell migration, and in the absence of filopodia, lamellipodia detect the stiffness of the surrounding ECM in a process called rigidity sensing. Several models have been proposed that describe this process. 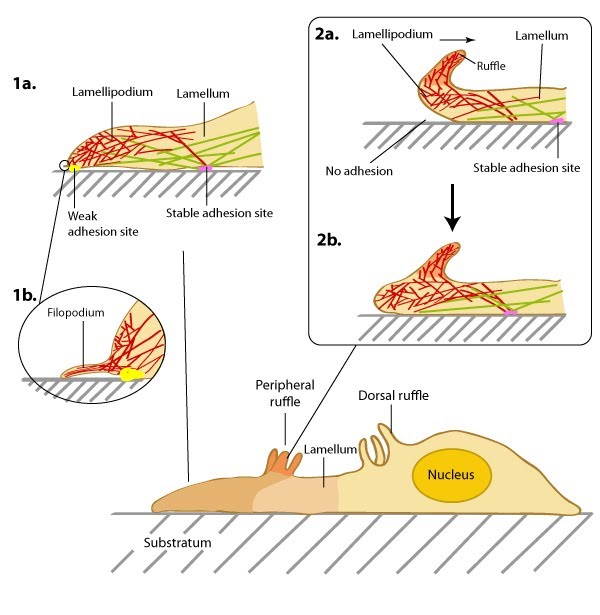 Figure 2. Structure of the lamellipodium and the lamellum: Actin filament assembly in the lamellipodium and retrograde movement of the filaments generates protrusive forces that drive cell motility in a specific direction. Lamellipodium (1a) and filopodium (1b) are common actin-based structures that are used to probe the cellular environment ahead of migrating cells. Adhesion to an underlying surface (ECM, other cells etc) determines the rate and direction of actin assembly during cell spreading and movement. During cell migration, nascent adhesions at the cell front ‘mature’ into stable adhesions as they progress towards the lamella. In the absence of adhesion at the leading edge (2a, 2b), the retrograde movement of actin filaments is converted into ruffles.Traditionally, it is believed that the lamellipodia and lamella are composed of two distinct actin networks [6], with the more dynamic lamellipodial actin lying on top of, and moving over, the more stable lamellal actin network [8]. A more recent model suggests maturing actin filaments, in the form of bundled ‘arcs’, form in the lamellipodia and are drawn back into the existing more stable actin filament network of the lamella. This is proposed to occur by the action of myosin motor proteins, especially myosin II, which was found to reside in the lamellipodia at a defined time point; specifically at the peak of protrusion, just preceding retraction [9]
Figure 2. Structure of the lamellipodium and the lamellum: Actin filament assembly in the lamellipodium and retrograde movement of the filaments generates protrusive forces that drive cell motility in a specific direction. Lamellipodium (1a) and filopodium (1b) are common actin-based structures that are used to probe the cellular environment ahead of migrating cells. Adhesion to an underlying surface (ECM, other cells etc) determines the rate and direction of actin assembly during cell spreading and movement. During cell migration, nascent adhesions at the cell front ‘mature’ into stable adhesions as they progress towards the lamella. In the absence of adhesion at the leading edge (2a, 2b), the retrograde movement of actin filaments is converted into ruffles.Traditionally, it is believed that the lamellipodia and lamella are composed of two distinct actin networks [6], with the more dynamic lamellipodial actin lying on top of, and moving over, the more stable lamellal actin network [8]. A more recent model suggests maturing actin filaments, in the form of bundled ‘arcs’, form in the lamellipodia and are drawn back into the existing more stable actin filament network of the lamella. This is proposed to occur by the action of myosin motor proteins, especially myosin II, which was found to reside in the lamellipodia at a defined time point; specifically at the peak of protrusion, just preceding retraction [9]
In each model, rigidity sensing and cell motility is achieved through myosin II induced periodic contractions of the lamellipodial actin network. Signals detected by adhesions located at the leading edge are transduced by the actin network to the rear of the lamellipodium where myosin II-dependent contractile forces are generated [10]. These forces subsequently pull at the lamellipodial actin network and result in variable migratory responses dependent on the strength of the adhesions.
In the traditional model, when adhesions are strong and the leading edge is anchored to the substrate, the cell pulls itself against the adhesion and moves forward in the process. The lamellipodial actin network continues to be pulled backwards, over the lamella, until it is severed from the initial leading edge adhesion. The generation of new actin filaments ensures lamellipodial growth and protrusion continues and new adhesions can be formed [8]. The retrograde movement of these filaments ensures myosin II remains in constant contact with the lamellipodial actin network. The duration of contraction is proportional to the width of the lamellipodium and in general, the more rigid the substrate, the stronger the signal, and the greater the contractile force generated [10]. Conversely when adhesions to the substrate are weak, contraction of the lamellipodial actin filament network will cause the lamellipodium to bend upwards, detaching the leading edge from the substrate surface. This results in ruffling and transient retraction [8]. Such events are characterized by an extension of the leading edge before retraction or ruffling occurs. Without strong adhesions for the cell to pull against the net forward movement is minimal. This type of pattern of migration has been noted in more slowly migrating cells, such as fibroblasts [11].
In the alternative model where actin filaments are gathered in the lamellipodia as ‘arcs’ and pulled back into the lamella by Myosin II, adhesions, and adhesion strength, play important roles in the rate of migration. In this model, where it is proposed that a single arc may contact a number of nascent focal adhesions, the adhesions, which form over a wider region, act both as a break to slow the flowing actin arcs, and as the site of future protrusion. If the adhesions are weak, they will slip backwards further into the lamella without any net gain in forward movement. If they are strong on the other hand it is proposed that they will slow rearward flowing actin arcs and thereby mark the region where the subsequent protrusion phases will commence. A net gain in forward movement will be achieved so long as newly forming adhesions at the leading edge remain strong [9].
In all models where adhesion strength determines the ability for mechanosensing and rate of cell motility, whether strong or weak, myosin-II is essential. How this protein is activated in this context is yet to be confirmed. MLCK (myosin light chain kinase) has been shown to be transported to the rear of the lamellipodium during the short window of time in between periodic contractions and so has been highlighted as a potential candidate for myosin II activation [10].
It should be noted that some evidence indicates lamellipodia are not required for cell motility. For example, when the formation of lamellipodia in epithelial cells was inhibited by the microinjection of skeletal muscle α-tropomyosin, leading edge protrusions and rapid cell migration was still observed [12]. A recent study supports such findings and suggests more complex mechanisms control cell motility. Specifically, the secretion of internalized components of the ECM via exocytosis, coupled to cortactin regulation of Arp2/3-mediated filament branching [13].
 Figure 2. Structure of the lamellipodium and the lamellum: Actin filament assembly in the lamellipodium and retrograde movement of the filaments generates protrusive forces that drive cell motility in a specific direction. Lamellipodium (1a) and filopodium (1b) are common actin-based structures that are used to probe the cellular environment ahead of migrating cells. Adhesion to an underlying surface (ECM, other cells etc) determines the rate and direction of actin assembly during cell spreading and movement. During cell migration, nascent adhesions at the cell front ‘mature’ into stable adhesions as they progress towards the lamella. In the absence of adhesion at the leading edge (2a, 2b), the retrograde movement of actin filaments is converted into ruffles.
Figure 2. Structure of the lamellipodium and the lamellum: Actin filament assembly in the lamellipodium and retrograde movement of the filaments generates protrusive forces that drive cell motility in a specific direction. Lamellipodium (1a) and filopodium (1b) are common actin-based structures that are used to probe the cellular environment ahead of migrating cells. Adhesion to an underlying surface (ECM, other cells etc) determines the rate and direction of actin assembly during cell spreading and movement. During cell migration, nascent adhesions at the cell front ‘mature’ into stable adhesions as they progress towards the lamella. In the absence of adhesion at the leading edge (2a, 2b), the retrograde movement of actin filaments is converted into ruffles.In each model, rigidity sensing and cell motility is achieved through myosin II induced periodic contractions of the lamellipodial actin network. Signals detected by adhesions located at the leading edge are transduced by the actin network to the rear of the lamellipodium where myosin II-dependent contractile forces are generated [10]. These forces subsequently pull at the lamellipodial actin network and result in variable migratory responses dependent on the strength of the adhesions.
In the traditional model, when adhesions are strong and the leading edge is anchored to the substrate, the cell pulls itself against the adhesion and moves forward in the process. The lamellipodial actin network continues to be pulled backwards, over the lamella, until it is severed from the initial leading edge adhesion. The generation of new actin filaments ensures lamellipodial growth and protrusion continues and new adhesions can be formed [8]. The retrograde movement of these filaments ensures myosin II remains in constant contact with the lamellipodial actin network. The duration of contraction is proportional to the width of the lamellipodium and in general, the more rigid the substrate, the stronger the signal, and the greater the contractile force generated [10]. Conversely when adhesions to the substrate are weak, contraction of the lamellipodial actin filament network will cause the lamellipodium to bend upwards, detaching the leading edge from the substrate surface. This results in ruffling and transient retraction [8]. Such events are characterized by an extension of the leading edge before retraction or ruffling occurs. Without strong adhesions for the cell to pull against the net forward movement is minimal. This type of pattern of migration has been noted in more slowly migrating cells, such as fibroblasts [11].
In the alternative model where actin filaments are gathered in the lamellipodia as ‘arcs’ and pulled back into the lamella by Myosin II, adhesions, and adhesion strength, play important roles in the rate of migration. In this model, where it is proposed that a single arc may contact a number of nascent focal adhesions, the adhesions, which form over a wider region, act both as a break to slow the flowing actin arcs, and as the site of future protrusion. If the adhesions are weak, they will slip backwards further into the lamella without any net gain in forward movement. If they are strong on the other hand it is proposed that they will slow rearward flowing actin arcs and thereby mark the region where the subsequent protrusion phases will commence. A net gain in forward movement will be achieved so long as newly forming adhesions at the leading edge remain strong [9].
In all models where adhesion strength determines the ability for mechanosensing and rate of cell motility, whether strong or weak, myosin-II is essential. How this protein is activated in this context is yet to be confirmed. MLCK (myosin light chain kinase) has been shown to be transported to the rear of the lamellipodium during the short window of time in between periodic contractions and so has been highlighted as a potential candidate for myosin II activation [10].
It should be noted that some evidence indicates lamellipodia are not required for cell motility. For example, when the formation of lamellipodia in epithelial cells was inhibited by the microinjection of skeletal muscle α-tropomyosin, leading edge protrusions and rapid cell migration was still observed [12]. A recent study supports such findings and suggests more complex mechanisms control cell motility. Specifically, the secretion of internalized components of the ECM via exocytosis, coupled to cortactin regulation of Arp2/3-mediated filament branching [13].
Video: Lateral waves of the lamellipodia. The extreme leading edge of mouse embryonic fibroblasts grown on fibronectin (viewed at 100X magnification). [ Michael Sheetz; Giannone, G. et al. Cell 2007,128 :561.]
Video: Migrating newt lung epithelial cells with fluorescent actin (yellow). Actin polymerization at the extreme leading edge of the lamellipodium and it’s subsequent retrograde movement towards the cell body. [Video uploaded to YouTube by CrawlingC3LL and produced by James I Lim at the Lawrence Berkeley National Laboratory, California, USA.]