Glossary Term: Intermediate Filaments (IFs)
Intermediate filaments (IFs) are a primary component of the cytoskeleton and extend throughout the cytoplasm and inner nuclear membrane. IF proteins form a large and diverse family that can be broadly grouped into five classes (see IF protein classes). However, IFs are not found in all eukaryotes and are absent in fungi and plants (as reviewed in [1]).
IF assembly begins with the folding of IF proteins into a conserved alpha-helical rod shape, followed by a series of polymerization and annealing events that lead to the formation of filaments roughly 8 to 12 nm in diameter (For further details see Dynamics of Assembly). Different IF combinations are found in different cell types, however not all IF classes will interact with each other. In contrast to other cytoskeletal components (e.g. actin filaments, microtubules), intermediate filaments lack polarity, are more stable and their constituent subunits do not bind nucleotides (such as ATP) (as reviewed in [2]).
Main Functions of IFs
- The tight association of protofilaments provide IFs with high tensile strength, making them the most stable component of the cytoskeleton. IFs can therefore be found in particularly durable structures such as hair, scales and fingernails.
- IFs create cell cohesion and prevent the acute fracture of epithelial cell sheets under tension. Extensive interactions between the constituent protofilaments of an IF make it resistant to compression, twisting, stretching and bending forces.
- IFs line the inner face of the nuclear envelope to help harness and protect the cell’s DNA.
- IFs help stabilize the extended axons of nerve cells.
Unique Features of Intermediate Filaments
1. IFs are heterogenous. When compared to actin and tubulin, there is greater sequence variation within IF genes and the proteins they produce. This yields more diversity in the types of structures they can form (e.g. hair, nails, feathers, horns). Although different IF protein classes share a common ancestral origin, IFs are only conserved in metazoans. Fungi and plants lack IFs and insects have only one class [3]. It seems likely that as the metazoan lineage evolved more intricate and complex tissues and organs, IFs were adapted and modified for tissue or cell specific purposes.2. IFs lack polarity. IF subunits do not bind nucleotides and are oriented in opposite directions during filament assembly. This leads to a similar structural appearance at both the filament ends, as suggested by FRAP (fluorescence recovery after photobleaching) experiments [4]. Furthermore IF subunit exchange is not confined to filament ends, as with microtubules and actin filaments, but occurs along their length [5].
3. IFs have high tensile strength and are resistant to compression, twisting and bending forces. The elastic nature of IFs is due the staggered assembly of IF subunits into protofilaments and the high degree of lateral versus longitudinal interactions within IFs (see Figure below). This structure promotes high tensile strength, toughness and long-range elasticity [6] (reviewed in [7]).
4. IFs lack motor proteins. Given IFs lack polarity it is not unexpected that no motor proteins have been identified to move along these structures [8].
5. IFs have slower filament dynamics. IFs exhibit a dynamic exchange of their constituent subunits, however this exchange rate is much slower than those observed with actin filaments or microtubules [5].
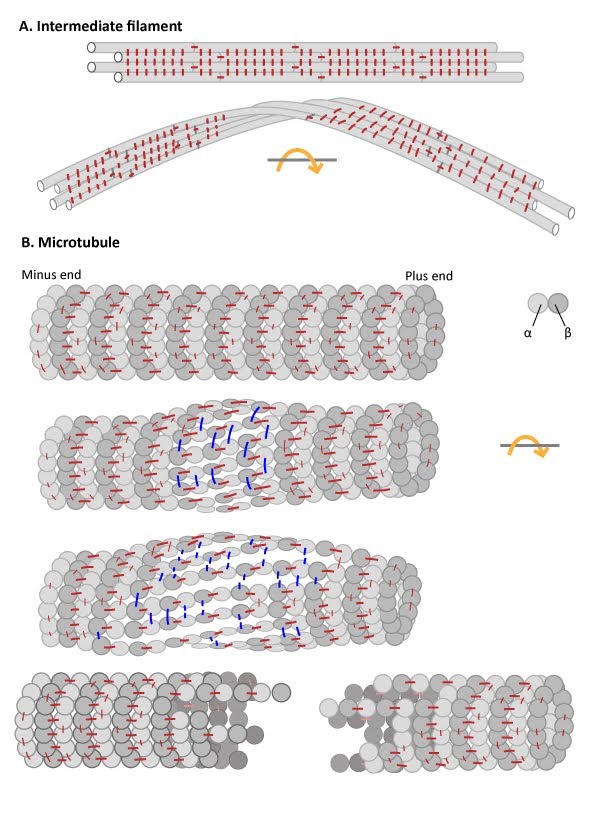
Figure: IFs are flexible and resistant to force. (A) Numerous lateral interactions and fewer longitudinal interactions between constituent protofilaments within an IF generates high tensile strength and makes IFs resistant to compression, bending, twisting and stretching forces. (B) Microtubules have more longitudinal interactions between constituent tubulin dimers within protofilaments and fewer lateral interactions between protofilaments. The stable longitudinal interactions within individual protofilaments promote rigidity within microtubules, making them resistant to bending and compression forces. In contrast, the weaker lateral interactions between protofilaments are susceptible to breakage when stressed by twisting forces.
Dynamics of Assembly
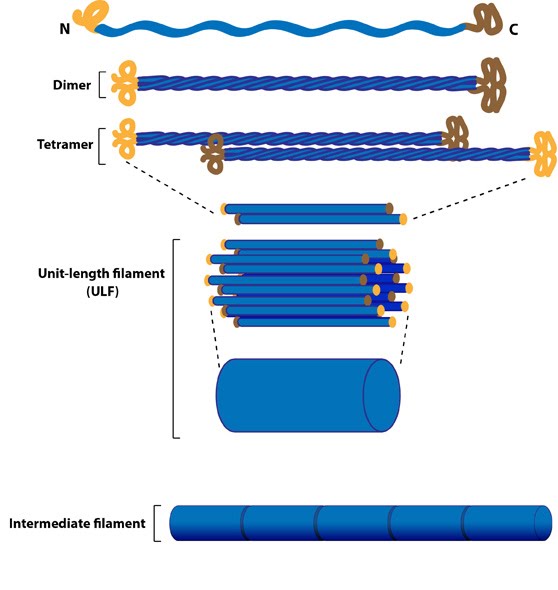
The soluble subunit for creating intermediate filaments is a tetramer. The tetramer is created from monomers in a stepwise fashion (see Figure below, as reviewed in [9]). First, two monomers associate via their central domains to form parallel helical coils around each other. This parallel dimer then associates with another parallel dimer in an antiparallel arrangement to form a staggered tetramer. The lateral association of eight tetramers results in the formation of a unit-length filaments (ULF). Two ULFs are able to anneal in an end-to-end fashion (i.e. longitudinally) to form a thick filament, approximately 16 nm in diameter. Further end-to-end annealing of ULFs results in filament elongation, which is followed by radial compaction to achieve the final intermediate filament diameter [5, 10].
Figure: Intermediate Filament Assembly. Intermediate filaments are built from monomers that associate with each other form dimers. Pairs of dimers then associate in an anti-parallel fashion to form staggered tetramers. Lateral associations between eight tetramers form unit-length filaments, which are able to anneal to each other, end-to-end, to form intermediate filaments.
IF protein classes, structure and functions
Type I and II: KeratinsKeratin proteins comprise the two largest classes of IF proteins. Historically, the two types of keratin were grouped as acidic (type I) or basic (type II) according to the overall physical properties of their composite amino acids. Keratin proteins first assemble into dimers, with one acidic and one basic chain, then into protofilaments and finally into IFs. In 2006, a universal nomenclature for each of the then known keratin genes and proteins, which totaled 54 (28 type I and 26 type II), was established to achieve international consensus for their naming and classification [11].
The expression of particular acidic and basic keratins can be cell type specific. Keratins are found in epithelial tissues and their expression can be altered during the lifetime of a cell. Keratins provide vital internal support and cohesion to epithelial cell sheets. For example, the basal layer of epithelial cells that constantly divide and give rise to new skin cells (keratinocytes) become filled with keratin filaments as they mature. The keratin filaments anchor the skin cells to the extracellular matrix (ECM) at their base and to adjacent cells at their sides, through structures called hemidesmosomes and desmosomes, respectively. As these skin cells die, the layer of dead cells form an essential barrier to water loss. Consequently, mutations in keratin genes are known to be responsible for a variety of skin diseases (as reviewed in [12]). Keratin-containing structures are also located external to the epithelial cell layer (e.g. hair and nails).
Type III: Desmin, vimentin
Type IV: Neurofilaments
Type V: Lamins