Contents
1.1 Role of Mechanobiology in Shaping Cells and Tissues 1.2 Common Themes In Mechanobiology 1.3 Types of Mechanosensing 1.4 Types of Forces Cells Encounter 1.5 The Dynamic Cytoskeleton 1.6 Common Features of Polymeric Cytoskeletal Systems 1.7 How Does the Cytoskeleton Transmit Mechanical Forces? Contribute | Essential Info: What is Mechanobiology?
|
References
- Tee SY., Bausch AR. & Janmey PA. The mechanical cell. Curr. Biol. 2009; 19(17):R745-8. [PMID: 19906576]
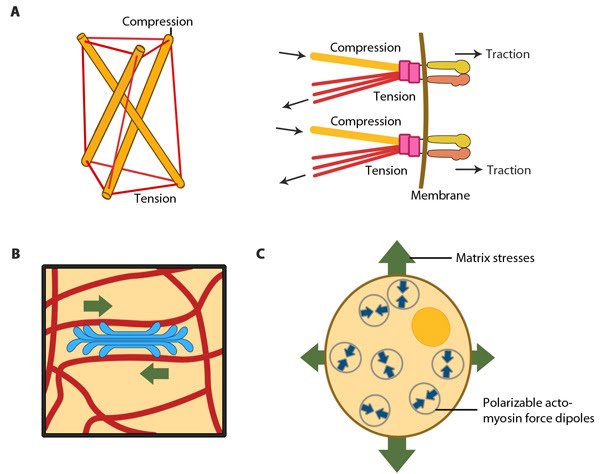
- Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J. Cell. Sci. 1993; 104 ( Pt 3):613-27. [PMID: 8314865]
- Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J. Cell. Sci. 2003; 116(Pt 7):1157-73. [PMID: 12615960]
- Wirtz D. Particle-tracking microrheology of living cells: principles and applications. Annu Rev Biophys 2009; 38:301-26. [PMID: 19416071]
- Gardel ML., Shin JH., MacKintosh FC., Mahadevan L., Matsudaira P. & Weitz DA. Elastic behavior of cross-linked and bundled actin networks. Science 2004; 304(5675):1301-5. [PMID: 15166374]
- Storm C., Pastore JJ., MacKintosh FC., Lubensky TC. & Janmey PA. Nonlinear elasticity in biological gels. Nature 2005; 435(7039):191-4. [PMID: 15889088]
- Koenderink GH., Dogic Z., Nakamura F., Bendix PM., MacKintosh FC., Hartwig JH., Stossel TP. & Weitz DA. An active biopolymer network controlled by molecular motors. Proc. Natl. Acad. Sci. U.S.A. 2009; 106(36):15192-7. [PMID: 19667200]
- Zemel A., Bischofs IB. & Safran SA. Active elasticity of gels with contractile cells. Phys. Rev. Lett. 2006; 97(12):128103. [PMID: 17026002]
- Zemel A., Rehfeldt F., Brown AE., Discher DE. & Safran SA. Cell shape, spreading symmetry and the polarization of stress-fibers in cells. J Phys Condens Matter 2010; 22(19):194110. [PMID: 20458358]
- Stamenovic D. & Ingber DE. Tensegrity-guided self assembly: from molecules to living cells. Soft Matter 2009; 5:1137–45. [DOI: 10.1039/B903916N]
- De R., Zemel A. & Safran SA. Theoretical concepts and models of cellular mechanosensing. Methods Cell Biol. 2010; 98:143-75. [PMID: 20816234]
- Ainsworth C. Cell biology: Stretching the imagination. Nature 2008; 456(7223):696-9. [PMID: 19079029]
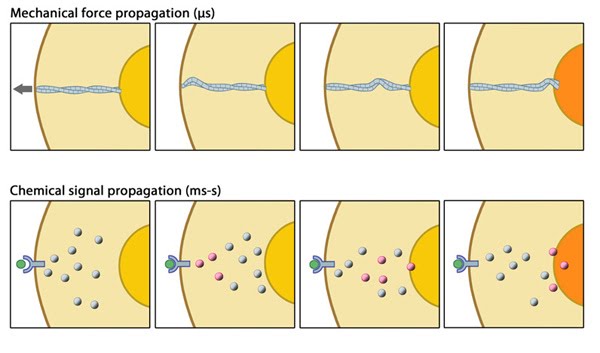
- Na S., Collin O., Chowdhury F., Tay B., Ouyang M., Wang Y. & Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc. Natl. Acad. Sci. U.S.A. 2008; 105(18):6626-31. [PMID: 18456839]
- DuFort CC., Paszek MJ. & Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011; 12(5):308-19. [PMID: 21508987]
- Wang N. & Suo Z. Long-distance propagation of forces in a cell. Biochem. Biophys. Res. Commun. 2005; 328(4):1133-8. [PMID: 15707995]
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006; 20(7):811-27. [PMID: 16675838]
- Maniotis AJ., Chen CS. & Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. U.S.A. 1997; 94(3):849-54. [PMID: 9023345]
- Pajerowski JD., Dahl KN., Zhong FL., Sammak PJ. & Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 2007; 104(40):15619-24. [PMID: 17893336]
- Hu S., Chen J., Fabry B., Numaguchi Y., Gouldstone A., Ingber DE., Fredberg JJ., Butler JP. & Wang N. Intracellular stress tomography reveals stress focusing and structural anisotropy in cytoskeleton of living cells. Am. J. Physiol., Cell Physiol. 2003; 285(5):C1082-90. [PMID: 12839836]
- Hu S., Eberhard L., Chen J., Love JC., Butler JP., Fredberg JJ., Whitesides GM. & Wang N. Mechanical anisotropy of adherent cells probed by a three-dimensional magnetic twisting device. Am. J. Physiol., Cell Physiol. 2004; 287(5):C1184-91. [PMID: 15213058]
- Wang N., Tytell JD. & Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009; 10(1):75-82. [PMID: 19197334]
- Ferrer JM., Lee H., Chen J., Pelz B., Nakamura F., Kamm RD. & Lang MJ. Measuring molecular rupture forces between single actin filaments and actin-binding proteins. Proc. Natl. Acad. Sci. U.S.A. 2008; 105(27):9221-6. [PMID: 18591676]
- Bustamante C., Chemla YR., Forde NR. & Izhaky D. Mechanical processes in biochemistry. Annu Rev Biochem. 2004; 73:705-48. [PMID: 15189157]
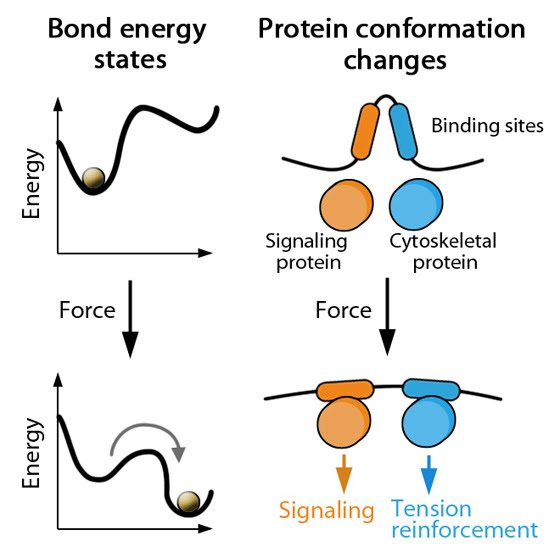
- Hoffman BD., Grashoff C. & Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature 2011; 475(7356):316-23. [PMID: 21776077]
- Evans EA. & Calderwood DA. Forces and bond dynamics in cell adhesion. Science 2007; 316(5828):1148-53. [PMID: 17525329]
- Chowdhury F., Na S., Collin O., Tay B., Li F., Tanaka T., Leckband DE. & Wang N. Is cell rheology governed by nonequilibrium-to-equilibrium transition of noncovalent bonds? Biophys. J. 2008; 95(12):5719-27. [PMID: 18835892]
- Thomas WE., Vogel V. & Sokurenko E. Biophysics of catch bonds. Annu Rev Biophys 2008; 37:399-416. [PMID: 18573088]