Read Further…
Syndecan localization and tissue specificity; Syndecan functions
For details on syndecan-4 signaling: Integrin β1 and syndecan-4 signaling in protrusion and adhesion dynamics
Related pages: Integrins; Cell-matrix adhesions; FA Initiation
For details on syndecan-4 signaling: Integrin β1 and syndecan-4 signaling in protrusion and adhesion dynamics
Related pages: Integrins; Cell-matrix adhesions; FA Initiation
Syndecans
are the only family of type I transmembrane proteoglycans with heparan
sulphate (HS) glycosaminoglycan (GAG) chains attached to them. They
function as co-receptors i.e. promote interactions of extracellular ligands with respective receptors and
fine-tune subsequent intracellular signals resulting in distinct
cellular functions (reviewed in [1, 2]). They have been implicated in
both cell-matrix and cell-cell adhesions (reviewed in [3]).
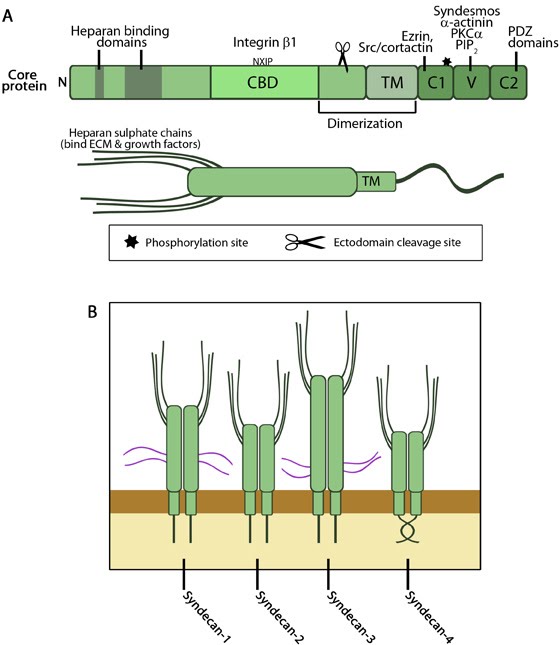
Atleast one syndecan is found in all multicellular organisms; mammals express four members (reviewed in [4, 5, 6, 7]), syndecans 1-4. Protein sizes range from 20 to 40kDa, mainly due to variable ectodomain lengths. The protein core consists of a N-terminal signal peptide, a distinct ecto (extracellular) domain with consensus sequences for GAG attachment and protease cleavage sites near the membrane, a transmembrane (TM) domain and a short C-terminal cytoplasmic tail. Of these, signaling of syndecan-4 has been substantially established followed by syndecans-1 and 2.
Figure: A. Domain structure of syndecans. Upper figure- The core protein domains are similar in all 4 members except for the length while proteins interacting with each domain could be member-specific. The ectodomain contains heparan sulphate (HS) binding domains and regions that directly or indirectly interact with integrin. The cell binding domain is defined in syndecan-4 and interacts with integrin β1 through the NXIP motif. Membrane proximal region has sequences recognized by proteases (scissor) for ectodomain shedding. The transmembrane spanning region is responsible for oligomerization along with residues in the membrane proximal region of ectodomain and is enhanced by C2 interactions. The cytoplasmic domain C1 mainly binds with proteins that link to actin cytoskeleton while C2 interacts with PDZ domain proteins that form submembranous complexes and regulate targeting and trafficking of cell surface molecules. V domain interactions with signaling proteins, especially PKCα, has been established in syndecan-4 and is regulated by PIP2 binding and the phosphorylation switch in C1 (star). Syndesmos is also syndecan-4 specific. Lower figure- Globular structure of syndecan proteoglycan with HS chains attached. These chains can bind a variety of ligands as listed in the text below. Adapted from [7, 8]. B. Members of syndecan family. Syndecans are always found as homodimers in vivo. Syndecans-1 and -3 proteins are larger than those of -2 and -4 and contain chondroitin sulphate chains (purple) in addition to HS chains. These chains provide them additional ligand binding capacity. The twisted clasp in the cytoplasmic domain of syndecan-4 is shown. Adapted from [9, 10].
Syndecans undergo regulated proteolytic cleavage at ectodomain sites near the membrane by matrix metalloproteinases and metzincins family of endoproteases (reviewed in [9]), a process called shedding, both as part of normal turnover as well as in response to external stimuli and is regulated by multiple pathways [19]. Besides disrupting syndecan signaling, the released soluble ectodomain acts as an antagonist to compete with intact syndecans for its ligands (reviewed in [20, 21]).
The TM domain drives oligomerization (homodimers or larger structures) through the GXXXG motif [22, 23], a prerequisite for syndecan signaling and function [24].
GAG chains: Besides HS chains, syndecans 1 and 3 also have chondroitin sulphate chains attached to the ectodomains towards the membrane, both through serine residues. Composition of these chains are reviewed in [11, 10]. The HS chains primarily bind heparan-binding motifs in a plethora of ligands (as mentioned above) and in addition, a wide range of bacterial and viral pathogens (reviewed in [13, 20]). Valency of HS chain-ligand interactions has been shown to be critical for syndecan-1 signaling in mediating adhesion [25]. Additionally, chain cleavage by heparanases can act as regulatory switch for ligand binding and signaling [26].
The distribution of the syndecan family members is tissue-specific and developmentally regulated. All cells but erythrocytes express at least one syndecan [41]. Syndecan-4 is commonly found in most tissues, co-expressed with at least one other syndecan. The distribution of other syndecans are listed in the table below [1, 2, 3].
However, altered levels or loss of syndecan expression in specific tissues can occur under certain pathophysiological conditions and can act as indicators of disease states such as tumor onset, progression and metastasis [9, 19, 20, 42].
1) ECM organization- ECM molecules such as fibronectin, vitronectin, laminins and fibrillin have heparan binding motif in addition to binding sites for other receptors. ECM assembly and contraction by syndecan-2 has been shown to have effect during embryo development (reviewed in [11, 13]).
2) Cytoskeleton organization- Syndecan-2 and -4 are known to affect stress fiber formation in an expression-dependent manner. Additionally, syndecan-4, in cooperation with integrin, is involved in cell spreading and focal adhesion formation/dynamics (reviewed in [13, 10]).
3) Ligand binding by shed ectodomain- Shed syndecan-1, -2 and -4 play a significant role in inflammation, development, wound healing and diseases, especially tumorigenesis by modulating angiogenesis and invasion ([42], reviewed in [13, 9, 20, 21, 10]).
Atleast one syndecan is found in all multicellular organisms; mammals express four members (reviewed in [4, 5, 6, 7]), syndecans 1-4. Protein sizes range from 20 to 40kDa, mainly due to variable ectodomain lengths. The protein core consists of a N-terminal signal peptide, a distinct ecto (extracellular) domain with consensus sequences for GAG attachment and protease cleavage sites near the membrane, a transmembrane (TM) domain and a short C-terminal cytoplasmic tail. Of these, signaling of syndecan-4 has been substantially established followed by syndecans-1 and 2.
Figure: A. Domain structure of syndecans. Upper figure- The core protein domains are similar in all 4 members except for the length while proteins interacting with each domain could be member-specific. The ectodomain contains heparan sulphate (HS) binding domains and regions that directly or indirectly interact with integrin. The cell binding domain is defined in syndecan-4 and interacts with integrin β1 through the NXIP motif. Membrane proximal region has sequences recognized by proteases (scissor) for ectodomain shedding. The transmembrane spanning region is responsible for oligomerization along with residues in the membrane proximal region of ectodomain and is enhanced by C2 interactions. The cytoplasmic domain C1 mainly binds with proteins that link to actin cytoskeleton while C2 interacts with PDZ domain proteins that form submembranous complexes and regulate targeting and trafficking of cell surface molecules. V domain interactions with signaling proteins, especially PKCα, has been established in syndecan-4 and is regulated by PIP2 binding and the phosphorylation switch in C1 (star). Syndesmos is also syndecan-4 specific. Lower figure- Globular structure of syndecan proteoglycan with HS chains attached. These chains can bind a variety of ligands as listed in the text below. Adapted from [7, 8]. B. Members of syndecan family. Syndecans are always found as homodimers in vivo. Syndecans-1 and -3 proteins are larger than those of -2 and -4 and contain chondroitin sulphate chains (purple) in addition to HS chains. These chains provide them additional ligand binding capacity. The twisted clasp in the cytoplasmic domain of syndecan-4 is shown. Adapted from [9, 10].
Extracellular domain
The ectodomains are capable of binding a variety of ligands including growth factors, cytokines, chemokines, morphogens, extracellular matrix proteins [11] and glycoproteins, cell adhesion receptors [12], enzymes, and other proteins (reviewed in [13]) through their GAG chains. Additionally, the cell binding domain (CBD) of the core protein can also act as ligands of integrin (via NXIP motif in syndecan-4) [12, 14, 15, 16, 17] and other receptors [18].Syndecans undergo regulated proteolytic cleavage at ectodomain sites near the membrane by matrix metalloproteinases and metzincins family of endoproteases (reviewed in [9]), a process called shedding, both as part of normal turnover as well as in response to external stimuli and is regulated by multiple pathways [19]. Besides disrupting syndecan signaling, the released soluble ectodomain acts as an antagonist to compete with intact syndecans for its ligands (reviewed in [20, 21]).
The TM domain drives oligomerization (homodimers or larger structures) through the GXXXG motif [22, 23], a prerequisite for syndecan signaling and function [24].
GAG chains: Besides HS chains, syndecans 1 and 3 also have chondroitin sulphate chains attached to the ectodomains towards the membrane, both through serine residues. Composition of these chains are reviewed in [11, 10]. The HS chains primarily bind heparan-binding motifs in a plethora of ligands (as mentioned above) and in addition, a wide range of bacterial and viral pathogens (reviewed in [13, 20]). Valency of HS chain-ligand interactions has been shown to be critical for syndecan-1 signaling in mediating adhesion [25]. Additionally, chain cleavage by heparanases can act as regulatory switch for ligand binding and signaling [26].
Cytoplasmic domains
The cytoplasmic tail contains highly conserved membrane-proximal C1 and distal C2 domains bordering a variable region (V) unique to each syndecan but conserved across species. These domains are implicated in the transport and targeting of syndecans to the membrane [27]. C1 has been shown to have affinity for tyrosine phosphate complexes (Src, Fyn, and tubulin) [28], dynamin II [29] (aids endocytosis) and signaling protein Rab5 30] (regulates ectodomain shedding). The conserved EFYA motif in C2 has binds class II PDZ domain ([31], reviewed in [10]), promote syndecan clustering and interactions with other proteins [32, 33]. Both C1 and C2 interactions are implicated in signaling internalization and recycling of both syndecans and the bound cargo (reviewed in [2]). The conserved residues in the V domain of syndecan-4 form a twisted clasp 34] and stabilize the oligomer by binding to PIP2, which is ablated by phosphorylation at Ser183 in C1 [35]<. Dimer stabilization is essential for recruitment and activation of protein kinase C (PKC) α to focal adhesions [36, 37], hence the phosphorylation acts as a regulatory switch. Additionally, V-domain binds α-actinin and syndesmos [38, 39]. Given their size, it is believed that the cytoplasmic domains may not bind partners simultaneously but according to external stimuli, thus act as sensors and trigger intracellular signals.Localization and tissue-specificity
Syndecans are known to localize to the basolateral surface in polarized cells [40]. A cell-binding motif in ectodomain of syndecan-4 mediates its unique localization to focal adhesions [12, 8].The distribution of the syndecan family members is tissue-specific and developmentally regulated. All cells but erythrocytes express at least one syndecan [41]. Syndecan-4 is commonly found in most tissues, co-expressed with at least one other syndecan. The distribution of other syndecans are listed in the table below [1, 2, 3].
Table: Tissue-specific localization of syndecan members
|
Syndecan member
|
Tissue |
| Syndecan-1 |
Epithelia |
| Syndecan-2 |
Endothelia and fibroblasts |
| Syndecan-3 |
Neural and some musculo-skeletal tissues |
However, altered levels or loss of syndecan expression in specific tissues can occur under certain pathophysiological conditions and can act as indicators of disease states such as tumor onset, progression and metastasis [9, 19, 20, 42].
Functions
Syndecans influence a range of physiological processes, the most conspicuous being wound healing. In conjunction with other receptors, especially integrins [43], syndecan signaling affects a variety of global cellular behaviors such as cell adhesion, migration and invasion by modulating the following:1) ECM organization- ECM molecules such as fibronectin, vitronectin, laminins and fibrillin have heparan binding motif in addition to binding sites for other receptors. ECM assembly and contraction by syndecan-2 has been shown to have effect during embryo development (reviewed in [11, 13]).
2) Cytoskeleton organization- Syndecan-2 and -4 are known to affect stress fiber formation in an expression-dependent manner. Additionally, syndecan-4, in cooperation with integrin, is involved in cell spreading and focal adhesion formation/dynamics (reviewed in [13, 10]).
3) Ligand binding by shed ectodomain- Shed syndecan-1, -2 and -4 play a significant role in inflammation, development, wound healing and diseases, especially tumorigenesis by modulating angiogenesis and invasion ([42], reviewed in [13, 9, 20, 21, 10]).