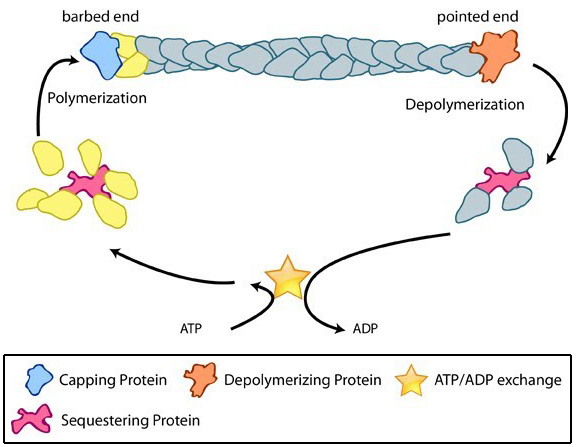
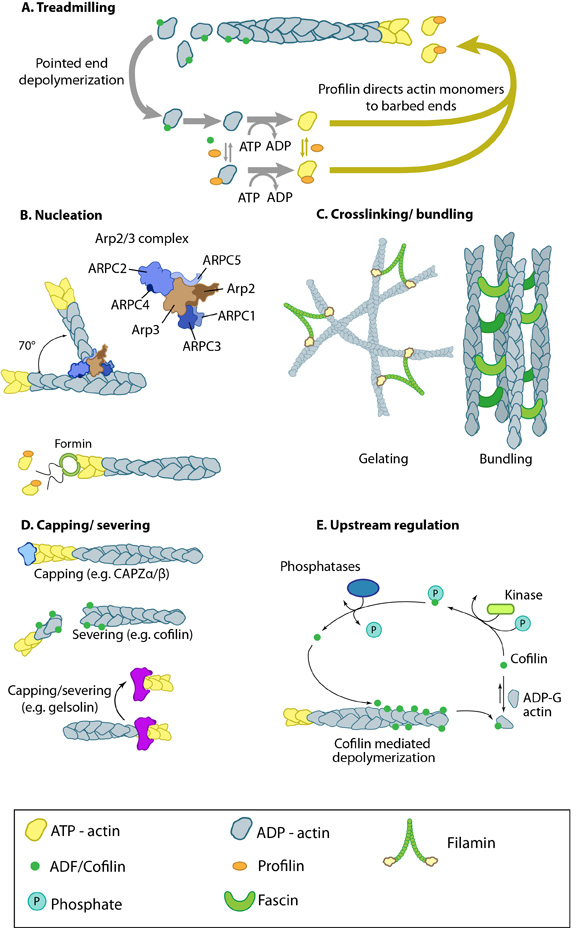
Quiz Test Your Knowledge! Click Here Contribute | Essential Info: Actin Filaments and Mechanotransduction4.3.1. Factors influencing actin filament length and treadmilling
|
References
- Carlier MF. & Pantaloni D. Control of actin dynamics in cell motility. J. Mol. Biol. 1997; 269(4):459-67. [PMID: 9217250]
- Pollard TD. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J. Cell Biol. 1986; 103(6 Pt 2):2747-54. [PMID: 3793756]
- Cooper JA. Effects of cytochalasin and phalloidin on actin. J. Cell Biol. 1987; 105(4):1473-8. [PMID: 3312229]
- Yarmola EG., Somasundaram T., Boring TA., Spector I. & Bubb MR. Actin-latrunculin A structure and function. Differential modulation of actin-binding protein function by latrunculin A. J. Biol. Chem. 2000; 275(36):28120-7. [PMID: 10859320]
- Morton WM, Ayscough KR, McLaughlin PJ. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat Cell Biol, 2000 Jun;2(6):376-8. [PMID: 10854330]
- Pollard TD. Introduction to actin and actin-binding proteins. In Guidebook to the Cytoskeletal and Motor Proteins, ed. Kreis RV, pp3-11. Oxford, UK: Oxford univ. Press. 2nd ed. ISBN: 0198599358
- Carlier MF., Jean C., Rieger KJ., Lenfant M. & Pantaloni D. Modulation of the interaction between G-actin and thymosin beta 4 by the ATP/ADP ratio: possible implication in the regulation of actin dynamics. Proc. Natl. Acad. Sci. U.S.A. 1993; 90(11):5034-8. [PMID: 8506348]
- Safer D., Sosnick TR. & Elzinga M. Thymosin beta 4 binds actin in an extended conformation and contacts both the barbed and pointed ends. Biochemistry 1997; 36(19):5806-16. [PMID: 9153421]
- Schutt CE., Myslik JC., Rozycki MD., Goonesekere NC. & Lindberg U. The structure of crystalline profilin-beta-actin. Nature 1993; 365(6449):810-6. [PMID: 8413665]
- Kang F, Purich DL, & Southwick FS. Profilin promotes barbed-end actin filament assembly without lowering the critical concentration. J Biol Chem 1999, 274(52):36963-36972. [PMID: 10601251]
- Athman R., Louvard D. & Robine S. The epithelial cell cytoskeleton and intracellular trafficking. III. How is villin involved in the actin cytoskeleton dynamics in intestinal cells? Am. J. Physiol. Gastrointest. Liver Physiol. 2002; 283(3):G496-502. [PMID: 12181160]