Actin Binding Proteins[Edit]
Many proteins and small molecule ligands bind to actin. In doing so they modulate the dynamics of the actin cytoskeleton, catalyze actin filament assembly or promote actin filament disassembly. This is not only integral to broader cellular processes such as cell migration and mechanosensing, but may also be exploited for experimental purposes. Proteins that bind to actin filaments direct the location, rate, and timing of actin filament assembly and disassembly. There is great structural diversity in the types of proteins which bind to actin, but the actin binding domains (ABD) themselves can be grouped according to the conserved structures they form (reviewed in [1]).
Examples of common actin binding domains include:
 Figure 1. Actin binding proteins influence actin dynamics: A. Treadmilling of actin filaments can be altered by profilin and ADF which generally increase and decrease the size of actin filaments, respectively. B. New filaments are nucleated by the ARP2/3 complex, which binds both G-actin monomers and the side of actin filaments to nucleate new filaments or branches. Formins nucleate new filaments by binding G-actin and through cooperation with profilin. C. Actin cross-linking proteins influence the packing and organization of actin filaments into secondary structures. D. Capping and severing proteins promote disassembly of actin filaments. E. Actin filament assembly can be modulated by events such as controlled nucleotide hydrolysis (e.g. ATP on actin) and reversible modifications (e.g. phosphorylation) on components that control actin assembly.
An example of how actin binding proteins may alter the dynamics and structure of the actin filament network is seen in the actin binding protein fascin which produces actin filament bundles during filopodia formation. Importantly, different types of crosslinking proteins will give rise to different types of filament based structures and will also modulate physical dynamics of the network to varying degrees (reviewed in [2, 3, 4]).
Figure 1. Actin binding proteins influence actin dynamics: A. Treadmilling of actin filaments can be altered by profilin and ADF which generally increase and decrease the size of actin filaments, respectively. B. New filaments are nucleated by the ARP2/3 complex, which binds both G-actin monomers and the side of actin filaments to nucleate new filaments or branches. Formins nucleate new filaments by binding G-actin and through cooperation with profilin. C. Actin cross-linking proteins influence the packing and organization of actin filaments into secondary structures. D. Capping and severing proteins promote disassembly of actin filaments. E. Actin filament assembly can be modulated by events such as controlled nucleotide hydrolysis (e.g. ATP on actin) and reversible modifications (e.g. phosphorylation) on components that control actin assembly.
An example of how actin binding proteins may alter the dynamics and structure of the actin filament network is seen in the actin binding protein fascin which produces actin filament bundles during filopodia formation. Importantly, different types of crosslinking proteins will give rise to different types of filament based structures and will also modulate physical dynamics of the network to varying degrees (reviewed in [2, 3, 4]).
Although functionally similar, other cross-linking proteins may promote the formation of larger networks, or filamentous structures, in different regions of the cell. Generally, proteins with similar functions (e.g. fascin, α-actinin) act cooperatively to enhance the mechanical integrity and responsiveness of the network [5] (reviewed in [6]).
- calponin-homology (CH) domain
- formin-homology-2 (FH2) domain
- WASp-homology-2 (WH2) domain
- actin-depolymerizing factor/cofilin (ADF/cofilin) domain
- gelsolin-homology domain
- myosin motor domain
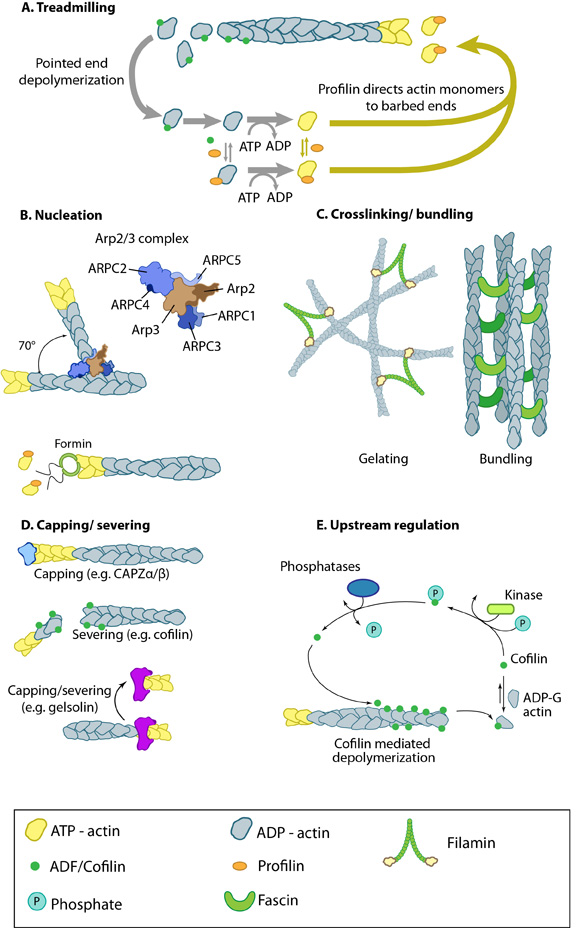 Figure 1. Actin binding proteins influence actin dynamics: A. Treadmilling of actin filaments can be altered by profilin and ADF which generally increase and decrease the size of actin filaments, respectively. B. New filaments are nucleated by the ARP2/3 complex, which binds both G-actin monomers and the side of actin filaments to nucleate new filaments or branches. Formins nucleate new filaments by binding G-actin and through cooperation with profilin. C. Actin cross-linking proteins influence the packing and organization of actin filaments into secondary structures. D. Capping and severing proteins promote disassembly of actin filaments. E. Actin filament assembly can be modulated by events such as controlled nucleotide hydrolysis (e.g. ATP on actin) and reversible modifications (e.g. phosphorylation) on components that control actin assembly.
Figure 1. Actin binding proteins influence actin dynamics: A. Treadmilling of actin filaments can be altered by profilin and ADF which generally increase and decrease the size of actin filaments, respectively. B. New filaments are nucleated by the ARP2/3 complex, which binds both G-actin monomers and the side of actin filaments to nucleate new filaments or branches. Formins nucleate new filaments by binding G-actin and through cooperation with profilin. C. Actin cross-linking proteins influence the packing and organization of actin filaments into secondary structures. D. Capping and severing proteins promote disassembly of actin filaments. E. Actin filament assembly can be modulated by events such as controlled nucleotide hydrolysis (e.g. ATP on actin) and reversible modifications (e.g. phosphorylation) on components that control actin assembly.Although functionally similar, other cross-linking proteins may promote the formation of larger networks, or filamentous structures, in different regions of the cell. Generally, proteins with similar functions (e.g. fascin, α-actinin) act cooperatively to enhance the mechanical integrity and responsiveness of the network [5] (reviewed in [6]).