Nucleation of Tubulin subunits[Edit]
Microtubule nucleation is unfavorable under normal conditions found in most living cells. Consequently, microtubules are nucleated from a complex of γ-tubulin and other protein components known as the γ-tubulin ring complex (γ-TuRC). γ-TuRC nucleates and caps the minus end of new filaments by providing stable binding sites for tubulin dimers [1]. Tubulin dimers primarily use longitudinal interactions to bind to each other and to γ−TuRC during the nucleation phase. As the protofilament length increases, lateral interactions between the protofilaments create additional stability that leads to a closed microtubule [2].
 Figure 1. Structure of the centrosome: In non-dividing cells, the centrosome, which is also known as the MTOC, consists of a pair of L-shaped centrioles and associated pericentriolar material. The ‘older’ of the two centrioles has additional proteins that form appendages along the exterior surface. The pericentriolar material contains numerous γ-TuRCs that nucleate the microtubule array. The centrioles have microtubules organized in a structure similar to the basal bodies found at the base of cilia and flagella. Figure adapted from [1]
Figure 1. Structure of the centrosome: In non-dividing cells, the centrosome, which is also known as the MTOC, consists of a pair of L-shaped centrioles and associated pericentriolar material. The ‘older’ of the two centrioles has additional proteins that form appendages along the exterior surface. The pericentriolar material contains numerous γ-TuRCs that nucleate the microtubule array. The centrioles have microtubules organized in a structure similar to the basal bodies found at the base of cilia and flagella. Figure adapted from [1]
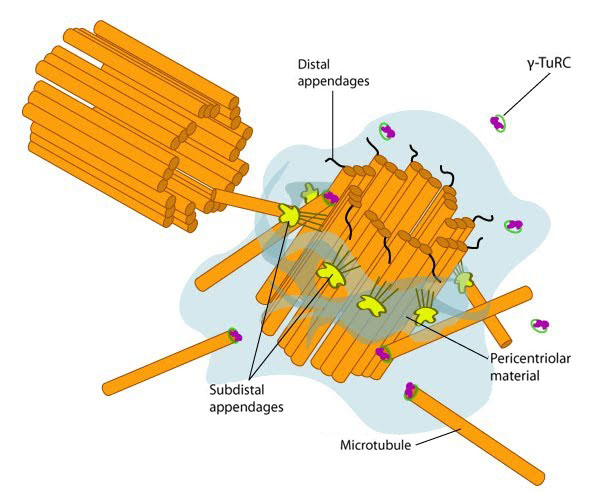 Figure 1. Structure of the centrosome: In non-dividing cells, the centrosome, which is also known as the MTOC, consists of a pair of L-shaped centrioles and associated pericentriolar material. The ‘older’ of the two centrioles has additional proteins that form appendages along the exterior surface. The pericentriolar material contains numerous γ-TuRCs that nucleate the microtubule array. The centrioles have microtubules organized in a structure similar to the basal bodies found at the base of cilia and flagella. Figure adapted from [1]
Figure 1. Structure of the centrosome: In non-dividing cells, the centrosome, which is also known as the MTOC, consists of a pair of L-shaped centrioles and associated pericentriolar material. The ‘older’ of the two centrioles has additional proteins that form appendages along the exterior surface. The pericentriolar material contains numerous γ-TuRCs that nucleate the microtubule array. The centrioles have microtubules organized in a structure similar to the basal bodies found at the base of cilia and flagella. Figure adapted from [1]After the slow nucleation phase, microtubules elongate rapidly. The minus end of γ-tubulin is anchored near the MTOC, whilst the plus end of γ-tubulin is exposed. This allows elongation to occur from the exposed γ-tubulin through interactions with the minus end of α/β-tubulin heterodimers [2]. Although it remains unclear whether the formation of longitudinal contacts with α-tubulin stimulates γ-tubulin hydrolysis of GTP, the rate of GTP hydrolysis on β-tubulin, along with its concentration are determining factors of microtubule assembly [2]. Once a tubulin dimer has been added to the lattice, the more likely it is for the GTP on β-tubulin to be hydrolyzed. MAPs can also control the rate of assembly/disassembly, the rate of GTP hydrolysis and the overall length of microtubules.