Cadherins[Edit]
This family of glycoproteins includes over 100 members divided into 6 subfamilies; type I classical cadherins, type II atypical cadherins, desmosomal cadherins, flamingo cadherins, proto-cadherins and several ungrouped members. Cadherins can be identified through common motifs in their extracellular domains termed cadherin repeats. Not all cadherins are involved in cell-cell adhesion, though type I and type II cadherins have well established roles in this process [1]. Both of these subfamilies contain cadherin repeats within their extracellular domains, with the outermost cadherin repeats facilitating extracellular interactions with cadherins on apposing cells (transinteractions). Type I cadherins can in addition engage in lateral interactions on the same cell (cis interactions)[2]. Intercellular interactions between cadherins can occur between those of the same type (homophilic binding) or a different type (heterophilic binding). Intracellular interactions involve the cytoplasmic domains of the cadherins. In the case of type I cadherins these interactions can be used to identify this subfamily, namely through their ability to bind catenins via their cytoplasmic tails. Catenins form part of the bridge connecting adherens junctions to the actin cytoskeleton. It should be noted that individual cadherin interactions are weak. The strength of cadherin-based adhesive junctions comes from the clustering of multiple, weak cadherin-cadherin interactions [3].
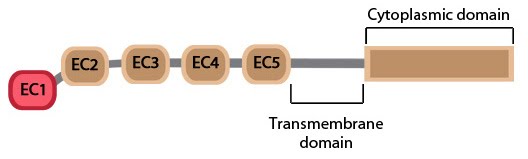
The cadherin protein family are common cell-adhesion molecules (CAMs) that mediate cell-cell contacts at anchoring junctions (e.g. adherens junctions, desmosomes) and at prominent sites of cell-cell communication (e.g. neuronal synapses). There are over 100 different cadherin family members that are grouped into at least 6 subfamilies, including type I classical cadherins, type II atypical cadherins and desmosomal cadherins [4]. All cadherins share a common architecture in their extracellular domain that comprises cadherin repeats, with classical cadherins containing five of these repeats (see Figure below). Subtle differences between cadherins impart each type with specificity for particular tissue and cell types. Cadherins use a common set of adaptor molecules and pathways to facilitate cell adhesion and communication, however the strength of adhesion varies with the type of cadherin present [5].
 Figure 2. Types of cadherin interactions: All cadherins have a common extracellular domain that is structured into tandem blocks that are variable in number and are called ‘cadherin repeats’; classical cadherins have five cadherin repeats (as shown). The cadherin intracellular domain is bound by adaptor proteins. (A) Example of a cis interaction (i.e. on the same cell) that is homophilic (i.e. cadherins of the same type). (B) Example of a trans interaction (i.e. between two cells) that is homophilic. (C) Example of a trans interaction that is heterophilic (between two different types of cadherin).
Most cadherins adhere by homophilic interactions (i.e. they bind to the same type of cadherin) but certain types (e.g. E-cadherin) also adhere by heterophilic interactions (i.e. they bind other types of cadherin). Cadherin association is sensitive to extracellular calcium (hence their name, calcium adhering). The interactions can take place laterally on the same cell, called a cis interaction, or between two cells, called a trans interaction (see Figure below).
Figure 2. Types of cadherin interactions: All cadherins have a common extracellular domain that is structured into tandem blocks that are variable in number and are called ‘cadherin repeats’; classical cadherins have five cadherin repeats (as shown). The cadherin intracellular domain is bound by adaptor proteins. (A) Example of a cis interaction (i.e. on the same cell) that is homophilic (i.e. cadherins of the same type). (B) Example of a trans interaction (i.e. between two cells) that is homophilic. (C) Example of a trans interaction that is heterophilic (between two different types of cadherin).
Most cadherins adhere by homophilic interactions (i.e. they bind to the same type of cadherin) but certain types (e.g. E-cadherin) also adhere by heterophilic interactions (i.e. they bind other types of cadherin). Cadherin association is sensitive to extracellular calcium (hence their name, calcium adhering). The interactions can take place laterally on the same cell, called a cis interaction, or between two cells, called a trans interaction (see Figure below).
Structurally, classical cadherins have five Ca+2-dependent extracellular domains and a relatively short cytoplasmic domain. Although cadherin-cadherin binding between the extracellular domains is relatively weak, the conformational changes that are induced after binding imparts the individual cadherins with rigidity. This stabilizes the interaction and fosters additional lateral cis interactions with other cadherins and generates tighter adhesions. Increased clustering of cadherins at sites of cell-cell contact correlates with increased stability and maturation of actin-based structures such as dendritic spines [4].
The classical cadherins (e.g. E-, N-, and P-cadherins) are the most common family members. Classical cadherins interact directly with p120ctn at their transmembrane region and through their cytoplasmic tails to beta (β)-catenin or plakoglobin (i.e. gamma [γ]-catenin). The correct function and stability of the cadherins requires these associations (reviewed in [6]). β-catenin binds tightly to classical cadherins before they are transported to the cell surface [7, 8]). Cadherins further interact indirectly with other adaptor proteins (e.g. alpha [α]-catenin, vinculin, EPLIN, α-actinin, zyxin) to form linkages between the cell membrane and the actin cytoskeleton (reviewed in [8]). Desmosomes in contrast, have two specialized cadherins that interact with specific adaptor proteins (e.g. plakoglobin, plakophilin, desmoplakin) to form links with the intermediate filaments.
Cadherin subfamilies:
* Type I classical cadherins – includes epithelial (E)-, neural (N)- and placental (P)-cadherin
* Type II atypical cadherins – includes vascular endothelial (VE)-cadherin
* Desmosomal cadherins – includes desmoglein and desmocollin
* Flamingo cadherins
* Proto-cadherins
The cadherin protein family are common cell-adhesion molecules (CAMs) that mediate cell-cell contacts at anchoring junctions (e.g. adherens junctions, desmosomes) and at prominent sites of cell-cell communication (e.g. neuronal synapses). There are over 100 different cadherin family members that are grouped into at least 6 subfamilies, including type I classical cadherins, type II atypical cadherins and desmosomal cadherins [4]. All cadherins share a common architecture in their extracellular domain that comprises cadherin repeats, with classical cadherins containing five of these repeats (see Figure below). Subtle differences between cadherins impart each type with specificity for particular tissue and cell types. Cadherins use a common set of adaptor molecules and pathways to facilitate cell adhesion and communication, however the strength of adhesion varies with the type of cadherin present [5].
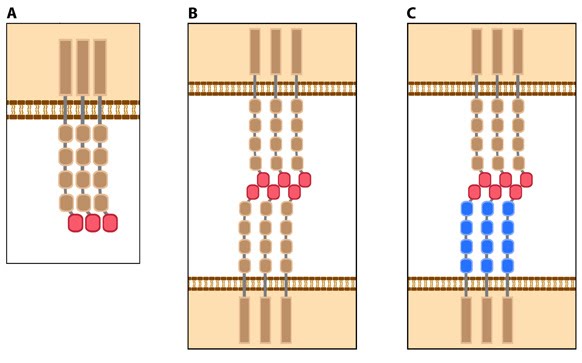 Figure 2. Types of cadherin interactions: All cadherins have a common extracellular domain that is structured into tandem blocks that are variable in number and are called ‘cadherin repeats’; classical cadherins have five cadherin repeats (as shown). The cadherin intracellular domain is bound by adaptor proteins. (A) Example of a cis interaction (i.e. on the same cell) that is homophilic (i.e. cadherins of the same type). (B) Example of a trans interaction (i.e. between two cells) that is homophilic. (C) Example of a trans interaction that is heterophilic (between two different types of cadherin).
Figure 2. Types of cadherin interactions: All cadherins have a common extracellular domain that is structured into tandem blocks that are variable in number and are called ‘cadherin repeats’; classical cadherins have five cadherin repeats (as shown). The cadherin intracellular domain is bound by adaptor proteins. (A) Example of a cis interaction (i.e. on the same cell) that is homophilic (i.e. cadherins of the same type). (B) Example of a trans interaction (i.e. between two cells) that is homophilic. (C) Example of a trans interaction that is heterophilic (between two different types of cadherin).Structurally, classical cadherins have five Ca+2-dependent extracellular domains and a relatively short cytoplasmic domain. Although cadherin-cadherin binding between the extracellular domains is relatively weak, the conformational changes that are induced after binding imparts the individual cadherins with rigidity. This stabilizes the interaction and fosters additional lateral cis interactions with other cadherins and generates tighter adhesions. Increased clustering of cadherins at sites of cell-cell contact correlates with increased stability and maturation of actin-based structures such as dendritic spines [4].
The classical cadherins (e.g. E-, N-, and P-cadherins) are the most common family members. Classical cadherins interact directly with p120ctn at their transmembrane region and through their cytoplasmic tails to beta (β)-catenin or plakoglobin (i.e. gamma [γ]-catenin). The correct function and stability of the cadherins requires these associations (reviewed in [6]). β-catenin binds tightly to classical cadherins before they are transported to the cell surface [7, 8]). Cadherins further interact indirectly with other adaptor proteins (e.g. alpha [α]-catenin, vinculin, EPLIN, α-actinin, zyxin) to form linkages between the cell membrane and the actin cytoskeleton (reviewed in [8]). Desmosomes in contrast, have two specialized cadherins that interact with specific adaptor proteins (e.g. plakoglobin, plakophilin, desmoplakin) to form links with the intermediate filaments.
Cadherin subfamilies:
* Type I classical cadherins – includes epithelial (E)-, neural (N)- and placental (P)-cadherin
* Type II atypical cadherins – includes vascular endothelial (VE)-cadherin
* Desmosomal cadherins – includes desmoglein and desmocollin
* Flamingo cadherins
* Proto-cadherins
* Ungrouped cadherins