Immunoglobulin superfamily (Ig) CAMs[Edit]
Members of this family include vascular and neural cell adhesions molecules (VCAM and NCAM), intercellular adhesion molecules (ICAM) and the nectins and nectin-like (Necl) proteins. Nectins in particular are involved in the formation of cadherin-based cell-cell junctions [1], mediating initial cell-cell contacts via nectin-nectin or nectin-Necl binding and establishing links to the actin cytoskeleton via nectin-afadin binding [2]. Of the four major groups of CAMs, IgCAMs are the only group that function independently of calcium.
The Ig superfamily is a large group of cell surface molecules that includes members such as:
 Figure 1. Schematic diagram of immunoglobulin superfamily members that are found in neurons: The extracytoplasmic portion of all Ig superfamily members is composed of a variable number of Ig domains (~3.7 nm per Ig domain) that together yield a final length of approximately 17-19 nm for both NCAM [3] and ICAM-1 [4]; certain Ig family members also contain a variable number of fibronectin III repeat domains. The transmembrane domain (TM) located at the extreme carboxy-terminus leaves a relatively small portion on the intracellular side for associating with other proteins. ICAM-1 is similar to ICAM-5 (not shown) but contains five Ig domains. Nectin and Necls share a similar structure, with three extracytoplasmic Ig-like domains (reviewed in [5])
Members of the Ig superfamily resemble each other in their three-dimensional structure as well as their amino acid sequence (reviewed in [6]). ICAMs and NCAMs form heterophilic and homophilic interactions (respectively) with adhesion molecules on other cells through a rigid extracytoplasmic rod domain that contains at least one flexible hinge domain [7, 8]. Although nectins and Necls form both heterophilic and homophilic interactions (reviewed in[1]), their homophilic interactions tend to be stronger [9].
Figure 1. Schematic diagram of immunoglobulin superfamily members that are found in neurons: The extracytoplasmic portion of all Ig superfamily members is composed of a variable number of Ig domains (~3.7 nm per Ig domain) that together yield a final length of approximately 17-19 nm for both NCAM [3] and ICAM-1 [4]; certain Ig family members also contain a variable number of fibronectin III repeat domains. The transmembrane domain (TM) located at the extreme carboxy-terminus leaves a relatively small portion on the intracellular side for associating with other proteins. ICAM-1 is similar to ICAM-5 (not shown) but contains five Ig domains. Nectin and Necls share a similar structure, with three extracytoplasmic Ig-like domains (reviewed in [5])
Members of the Ig superfamily resemble each other in their three-dimensional structure as well as their amino acid sequence (reviewed in [6]). ICAMs and NCAMs form heterophilic and homophilic interactions (respectively) with adhesion molecules on other cells through a rigid extracytoplasmic rod domain that contains at least one flexible hinge domain [7, 8]. Although nectins and Necls form both heterophilic and homophilic interactions (reviewed in[1]), their homophilic interactions tend to be stronger [9].
- vascular cell adhesion molecules (VCAM)
- neural cell adhesion molecules (NCAM)
- intercellular adhesion molecules (ICAM)
- nectin and nectin-like (Necl) family
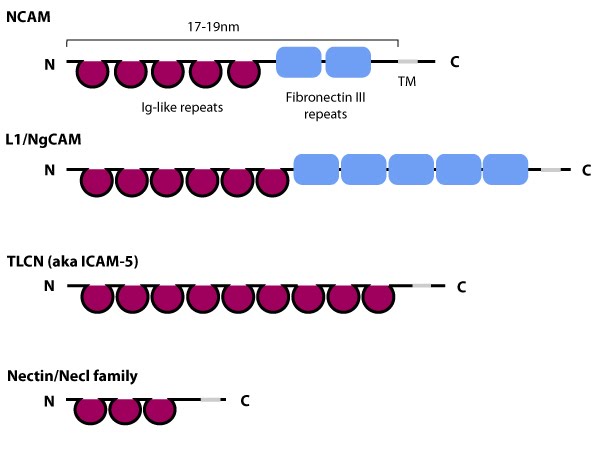 Figure 1. Schematic diagram of immunoglobulin superfamily members that are found in neurons: The extracytoplasmic portion of all Ig superfamily members is composed of a variable number of Ig domains (~3.7 nm per Ig domain) that together yield a final length of approximately 17-19 nm for both NCAM [3] and ICAM-1 [4]; certain Ig family members also contain a variable number of fibronectin III repeat domains. The transmembrane domain (TM) located at the extreme carboxy-terminus leaves a relatively small portion on the intracellular side for associating with other proteins. ICAM-1 is similar to ICAM-5 (not shown) but contains five Ig domains. Nectin and Necls share a similar structure, with three extracytoplasmic Ig-like domains (reviewed in [5])
Figure 1. Schematic diagram of immunoglobulin superfamily members that are found in neurons: The extracytoplasmic portion of all Ig superfamily members is composed of a variable number of Ig domains (~3.7 nm per Ig domain) that together yield a final length of approximately 17-19 nm for both NCAM [3] and ICAM-1 [4]; certain Ig family members also contain a variable number of fibronectin III repeat domains. The transmembrane domain (TM) located at the extreme carboxy-terminus leaves a relatively small portion on the intracellular side for associating with other proteins. ICAM-1 is similar to ICAM-5 (not shown) but contains five Ig domains. Nectin and Necls share a similar structure, with three extracytoplasmic Ig-like domains (reviewed in [5])