α-catenin[Edit]
α-catenin has broad activity that contributes to processes such as differentiation (i.e. commitment to a particular cell type), embryonic and tissue development, and cell migration (reviewed in [1, 2]). α-catenin is concentrated at cell-cell adhesion sites, e.g., tight junctions [3], and adherens junctions (reviewed in [4]), through its association with a related family member, beta (β)-catenin; this binding interaction is controlled by phosphorylation of either α- or β-catenin [5, 6] (reviewed in [7]) and phosphorylated β-catenin is expected to compete with homodimerziation of α-catenin [5, 8]. Dimerization of α-catenin creates a complex with functional domains at both ends that preferentially binds actin filaments, in contrast to the monomer which prefers E-cadherin-β-catenin complexes [9].
 Figure 1. Alpha (α)-catenin: This schematic diagram illustrates the molecular organization of
α-catenin and provides examples for how α-catenin is represented in
figures throughout this resource.Because β-catenin binds tightly to classical cadherins before they are transported to the cell surface [10, 11]), it was originally suggested that α-catenin formed an indirect link between adhesion receptors and the actin cytoskeleton via its association with β-catenin or other actin-binding proteins (e.g. vinculin and α-actinin) [11]. However, later work showed that α-catenin cannot bind the E-cadherin-β-catenin complex and actin simultaneously, nor does it bind actin indirectly through its binding partners, vinculin or α-actinin [12]. Thus, rather than serving as a bridge to the cytoskeleton, α-catenin appears to function primarily as a molecular switch that promotes stable cell-cell adhesions (e.g. adherens junctions).
Figure 1. Alpha (α)-catenin: This schematic diagram illustrates the molecular organization of
α-catenin and provides examples for how α-catenin is represented in
figures throughout this resource.Because β-catenin binds tightly to classical cadherins before they are transported to the cell surface [10, 11]), it was originally suggested that α-catenin formed an indirect link between adhesion receptors and the actin cytoskeleton via its association with β-catenin or other actin-binding proteins (e.g. vinculin and α-actinin) [11]. However, later work showed that α-catenin cannot bind the E-cadherin-β-catenin complex and actin simultaneously, nor does it bind actin indirectly through its binding partners, vinculin or α-actinin [12]. Thus, rather than serving as a bridge to the cytoskeleton, α-catenin appears to function primarily as a molecular switch that promotes stable cell-cell adhesions (e.g. adherens junctions).
Current models suggest that α-catenin promotes stronger adhesions in a few ways: 1) α-catenin may foster lateral clustering and activation of cadherins [13]; 2) α-catenin may recruit formins at nascent cell-cell contacts to produce new filaments that push against and bring the membranes together [14] (reviewed in [1, 2]); and 3) α-catenin may regulate membrane protrusive activity [15] and suppress Arp2/3 complex-mediated actin nucleation and polymerization at the leading edge in the lamellipodium [9] (reviewed in [2]).
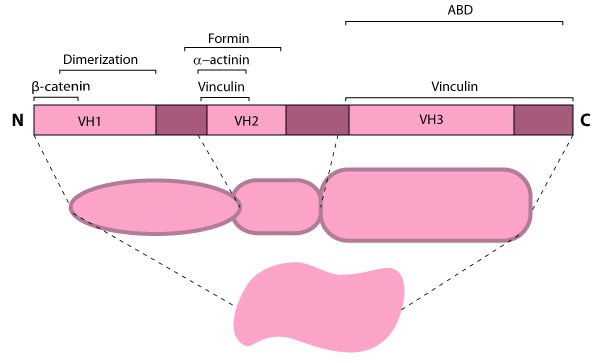 Figure 1. Alpha (α)-catenin: This schematic diagram illustrates the molecular organization of
α-catenin and provides examples for how α-catenin is represented in
figures throughout this resource.
Figure 1. Alpha (α)-catenin: This schematic diagram illustrates the molecular organization of
α-catenin and provides examples for how α-catenin is represented in
figures throughout this resource.Current models suggest that α-catenin promotes stronger adhesions in a few ways: 1) α-catenin may foster lateral clustering and activation of cadherins [13]; 2) α-catenin may recruit formins at nascent cell-cell contacts to produce new filaments that push against and bring the membranes together [14] (reviewed in [1, 2]); and 3) α-catenin may regulate membrane protrusive activity [15] and suppress Arp2/3 complex-mediated actin nucleation and polymerization at the leading edge in the lamellipodium [9] (reviewed in [2]).