Parvin[Edit]
Adaptor proteins Other proteins located at sites of cell adhesion include the adaptor proteins which connect the adhesion molecules to the cytoskeleton and signaling molecules. Examples include parvin, paxillin, talin, tensin, vinculin and zyxin.
 Figure 1. Parvin: This schematic diagram illustrates the molecular organization of parvin and provides examples for how parvin is presented in figures throughout this resource. Relevant domains believed to be important for binding to actin (e.g. CH1, CH2) and protein-protein interactions are highlighted (reviewed in [1])Although CH domains are a common feature shared between members of the α-actinin superfamily (reviewed in [3]), the ABDs of parvin are unique and more closely related to fimbrin, which further separates the parvins into a separate family within the α-actinin superfamily [2]. Furthermore, the CH domains of parvin were suggested to have evolved for specifically interacting with non-actin targets at sites of focal adhesion assembly; for example, recruitment of parvin to focal adhesions (FAs) requires an association with paxillin via a paxillin-binding sequence (PBS) motif contained within second CH domain of parvin [4]. Similar to other members of the α-actinin superfamily, α-parvin (aka actopaxin) may function as a dimer [5].
Figure 1. Parvin: This schematic diagram illustrates the molecular organization of parvin and provides examples for how parvin is presented in figures throughout this resource. Relevant domains believed to be important for binding to actin (e.g. CH1, CH2) and protein-protein interactions are highlighted (reviewed in [1])Although CH domains are a common feature shared between members of the α-actinin superfamily (reviewed in [3]), the ABDs of parvin are unique and more closely related to fimbrin, which further separates the parvins into a separate family within the α-actinin superfamily [2]. Furthermore, the CH domains of parvin were suggested to have evolved for specifically interacting with non-actin targets at sites of focal adhesion assembly; for example, recruitment of parvin to focal adhesions (FAs) requires an association with paxillin via a paxillin-binding sequence (PBS) motif contained within second CH domain of parvin [4]. Similar to other members of the α-actinin superfamily, α-parvin (aka actopaxin) may function as a dimer [5].
Parvin
The parvins are a family of actin binding proteins (known as α-, β- and γ-parvin in mammals) that are members of the actin linking functional module at cell-matrix adhesion sites (reviewed in [1]). Parvin is a small protein (42 kDa) that contains a variable amino terminus followed by two actin-binding domains (ABDs) each composed from two calponin homology (CH) domains [2].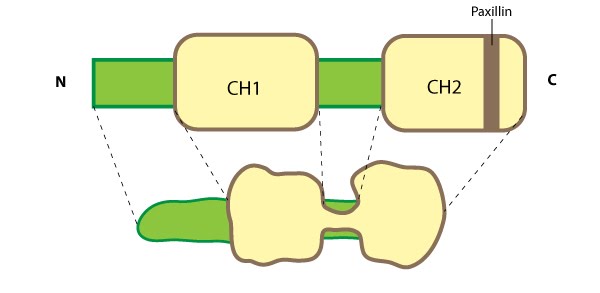 Figure 1. Parvin: This schematic diagram illustrates the molecular organization of parvin and provides examples for how parvin is presented in figures throughout this resource. Relevant domains believed to be important for binding to actin (e.g. CH1, CH2) and protein-protein interactions are highlighted (reviewed in [1])
Figure 1. Parvin: This schematic diagram illustrates the molecular organization of parvin and provides examples for how parvin is presented in figures throughout this resource. Relevant domains believed to be important for binding to actin (e.g. CH1, CH2) and protein-protein interactions are highlighted (reviewed in [1])Parvin is found in several cell types and at many locations in the cell such as: the leading edge of migrating cells and at sites of growing adhesions; it extends from mature FAs; and it partially localizes with stress fibers [2, 4]. Parvin co-localizes completely with talin in FAs and with fibers along the cell body [2]. As parvin is not usually found along the entire length of stress fibers [4], these central fibers more likely resemble tensin-rich fibrillar adhesions (FBs) [6]. Parvin is a member of a triad known as IPP (ILK-PINCH-parvin) which controls the maturation of cell-matrix adhesions by forming a permissive platform for tensin recruitment [7]. Parvin contains numerous potential phosphorylation consensus sequences for kinases such as protein kinase C [4] and extracellular signal-regulated protein kinase [8]; phosphoryation of parvin increases during cell adhesion/spreading [8].