Tensin[Edit]
Tensin is a cytoskeleton scaffolding protein that was named for its ability to form a bridge that maintains tension between the actin filaments and cell-matrix adhesion sites (reviewed in [1]).
 Figure 1. Tensin: This
schematic diagram illustrates the molecular organization of tensin and
provides examples for how tensin is represented in figures throughout
this resource. Relevant domains believed to be important for binding to actin and for protein-protein interactions are highlighted (reviewed in [1, 2]). PIP2- phosphatidylinositol [3, 4]-bis-phosphate; YP- phosphotyrosine; SH2- Src homology 2.
Figure 1. Tensin: This
schematic diagram illustrates the molecular organization of tensin and
provides examples for how tensin is represented in figures throughout
this resource. Relevant domains believed to be important for binding to actin and for protein-protein interactions are highlighted (reviewed in [1, 2]). PIP2- phosphatidylinositol [3, 4]-bis-phosphate; YP- phosphotyrosine; SH2- Src homology 2.
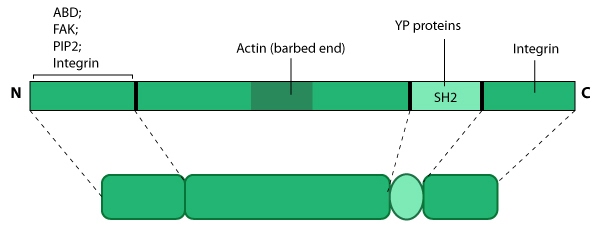 Figure 1. Tensin: This
schematic diagram illustrates the molecular organization of tensin and
provides examples for how tensin is represented in figures throughout
this resource. Relevant domains believed to be important for binding to actin and for protein-protein interactions are highlighted (reviewed in [1, 2]). PIP2- phosphatidylinositol [3, 4]-bis-phosphate; YP- phosphotyrosine; SH2- Src homology 2.
Figure 1. Tensin: This
schematic diagram illustrates the molecular organization of tensin and
provides examples for how tensin is represented in figures throughout
this resource. Relevant domains believed to be important for binding to actin and for protein-protein interactions are highlighted (reviewed in [1, 2]). PIP2- phosphatidylinositol [3, 4]-bis-phosphate; YP- phosphotyrosine; SH2- Src homology 2.Tensin contains three actin-binding domains (ABDs) that allows it to form crosslinks along actin filaments; it also prevents actin assembly by capping actin filaments at the barbed end [5, 6]. Tensin has numerous phosphorylation sites and multiple protein interaction domains for both structural components (e.g. paxillin, β-integrin [7]) and signaling molecules (e.g. Src, phosphatidylinositol 3-kinase [PI3K], focal adhesion kinase [FAK]) [8] (reviewed in [2]). Phosphorlyation of tensin corresponds with cell-ECM binding [9] and growth factor stimulation [10] (reviewed in [1]). Tensin forms a C-shaped structure [11] that binds focal adhesion components at both ends [12]. Tensin is also proposed to form a dimer via its carboxy-terminus and this association may be dependent upon its phosphorylation state [11].