Vinculin[Edit]
Vinculin is a key factor that couples, transmits, transduces, and regulates mechanical force between the cytoskeleton and adhesion receptors (reviewed in [1]).
 Figure 1. Vinculin: This schematic diagram illustrates the molecular organization of vinculin and shows how vinculin is represented throughout this resource. Relevant domains believed to be important for actin binding and protein-protein interactions are highlighted (reviewed in [2]).Vinculin frequently links adhesion receptors (e.g. integrins) to the contractile actin–myosin cytoskeleton by binding either talin through its amino-terminal globular head domain [3], or paxillin through its rod-like tail domain [4]. Vinculin can also bind to lipids through the tail domain. The head and tail domains are linked by a flexible hinge that also contains binding sites for components of the actin polymerizing module (e.g. Arp2/3 complex [12473693], Ena/VASP proteins [5, 6]).
Figure 1. Vinculin: This schematic diagram illustrates the molecular organization of vinculin and shows how vinculin is represented throughout this resource. Relevant domains believed to be important for actin binding and protein-protein interactions are highlighted (reviewed in [2]).Vinculin frequently links adhesion receptors (e.g. integrins) to the contractile actin–myosin cytoskeleton by binding either talin through its amino-terminal globular head domain [3], or paxillin through its rod-like tail domain [4]. Vinculin can also bind to lipids through the tail domain. The head and tail domains are linked by a flexible hinge that also contains binding sites for components of the actin polymerizing module (e.g. Arp2/3 complex [12473693], Ena/VASP proteins [5, 6]).
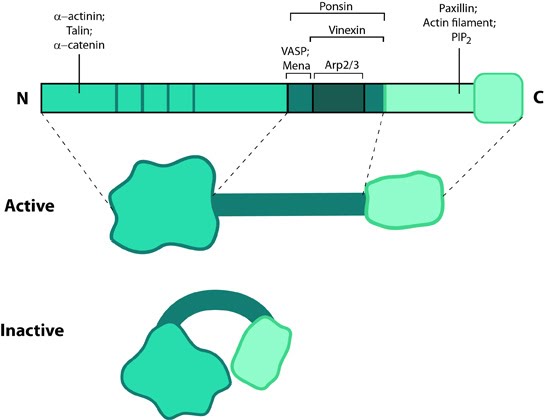 Figure 1. Vinculin: This schematic diagram illustrates the molecular organization of vinculin and shows how vinculin is represented throughout this resource. Relevant domains believed to be important for actin binding and protein-protein interactions are highlighted (reviewed in [2]).
Figure 1. Vinculin: This schematic diagram illustrates the molecular organization of vinculin and shows how vinculin is represented throughout this resource. Relevant domains believed to be important for actin binding and protein-protein interactions are highlighted (reviewed in [2]).Activation
Vinculin generally forms two structural states, an open (active) and closed (inactive) state, which are controlled by interaction(s) between the head and tail domains [7, 8]. Whether vinculin can bind to other factors depends both allosterically and sterically on the formation of the complete open state (reviewed in [2]). This in turn is favored by combinatorial binding of ligands namely talin, phosphatidylinositol 4,5-bis-phosphate [PIP2] and actin [8, 9, 10, 11](reviewed in [1]). Phosphorylation at 4 residues has been proposed to prime vinculin for this complex formation and hence the activation process [12]. Activation of vinculin influences its ability to form oligomers or other complxes in cells [13, 14].Localization
574428]; later work showed it was present in all adherens junctions [15]. Lipid binding regulates its placement in the adhesions as well as disassembly hence stimulating motility (reviewed in [2]).Vinculin recruitment to adhesion sites is mechanically regulated by ligand binding(e.g. other cells, extracellullar matrix) and activation of adhesion receptors. Physical restructuring of adhesion receptors such as integrins and their linked mechanosensors (e.g. talin) is transmitted to the cell interior to stimulate contraction of actin stress fibers; this promotes vinculin binding to these sites [3] and subsequent ordering of vinculin domains (reviewed in [8]). Interestingly, reduced cellular tension doesn’t lead to altered vinculin binding as has been observed for other structural components (e.g. zyxin [16]).
Functions
Vinculin N-terminal, when bound to talin, partially opens up and aids integrin clustering for FA growth, possibly by retaining the activated state of integrins [17, 18]. The C-terminal forms a mechanosensitive link between adhesion receptors and the actin cytoskeleton to help recruit other components of the actin linking module (e.g. α-actinin, paxillin) and influence the mechanical strength of the cell [3, 18, 19]. Additionally, vinculin possesses actin filament capping activity [20]. This needs an complete opening of vinculin structure allowing C-terminal of the tail to compete with formin mDia1 for actin barbed ends.Vinculin also contributes to stability of focal adhesion under high forces by regulating contractile stress generation [3], thereby influencing the cell migration speed [21]. Cells deficient in vinculin cannot form lamellipodia, assemble stress fibers, or spread efficiently over a substrate [18].