Introduction to integrin and its structure[Edit]
Integrins are proteins that function mechanically, by attaching the cell cytoskeleton to the extracellular matrix (ECM), and biochemically, by sensing whether adhesion has occurred. The integrin family of proteins consists of alpha and beta subtypes, which form transmembrane heterodimers. Integrins function as adhesion receptors for extracellular ligands and transduce biochemical signals into the cell, through downstream effector proteins. Remarkably, they function bidirectionally, meaning they can transmit information both outside-in and inside-out (reviewed in [1, 2]).
 Figure 1. Integrin alpha chain: Integrin α subunit domains: Top: Linear domain arrangement. Middle: The globular structure formed by protein domains. Bottom: simplified version of the integrin α subunit. The αI domain is present in some subtypes of the α subunit.
Each integrin heterodimer consists of an alpha (α) and a beta (β) subunit associated by noncovalent interactions forming an extracellular ligand-binding head, two multi-domain `legs’, two single-pass transmembrane helices and two short cytoplasmic tails. The α and β groups show no homology to each other,however, conserved regions are found among subtypes of both groups.
Figure 1. Integrin alpha chain: Integrin α subunit domains: Top: Linear domain arrangement. Middle: The globular structure formed by protein domains. Bottom: simplified version of the integrin α subunit. The αI domain is present in some subtypes of the α subunit.
Each integrin heterodimer consists of an alpha (α) and a beta (β) subunit associated by noncovalent interactions forming an extracellular ligand-binding head, two multi-domain `legs’, two single-pass transmembrane helices and two short cytoplasmic tails. The α and β groups show no homology to each other,however, conserved regions are found among subtypes of both groups.
The α subunit leg consists of a thigh and 2 calf domains that support the ligand binding head formed by a β-propeller domain with 7 repeats forming the blades (shown as a cylinder in the figure below). Some of the propeller blade domains contain calcium binding EF-hand domains on the lower side; these allosterically affect ligand binding [3]. An additional αI (interactive) domain containing ~200 residues is present in some vertebrate α chains [4] (nine human α subtypes) between the propeller repeats 2 and 3 [5]. This contains a metal-ion dependent adhesion site (MIDAS) which is important for ligand binding.
 Figure 2. Integrin beta chain: Integrin β subunit domains: Top: Linear domain arrangement. Middle: The globular structure formed by protein domains. Bottom: simplified version of the integrin β subunit.
The β subunit comprises of 4 cysteine-rich epidermal growth factor (EGF) repeats, a hybrid domain (split in sequence), an I-like domain (βI) and a plexin-sempahorin-integrin (PSI) domain. Similar to αI, the contains βI domain contains a MIDAS site for ligand binding and additional regulatory site “adjacent to MIDAS” or ADMIDAS, inhibited by Ca2+ and activated by Mn2+ [3] for ligand binding.
Figure 2. Integrin beta chain: Integrin β subunit domains: Top: Linear domain arrangement. Middle: The globular structure formed by protein domains. Bottom: simplified version of the integrin β subunit.
The β subunit comprises of 4 cysteine-rich epidermal growth factor (EGF) repeats, a hybrid domain (split in sequence), an I-like domain (βI) and a plexin-sempahorin-integrin (PSI) domain. Similar to αI, the contains βI domain contains a MIDAS site for ligand binding and additional regulatory site “adjacent to MIDAS” or ADMIDAS, inhibited by Ca2+ and activated by Mn2+ [3] for ligand binding.
i) Ligand binding
The βI domain binds ligand together with the β-propeller or with αI (if present) through MIDAS in a Mg2+ dependent fashion [6] at the interface in the headpiece. While Asp carboxyl group coordinates the βI MIDAS ion Mg2+, side chain hydrogen of the Arg of the RGD ligand binds directly to the Asp in domains 2 and 3 of β-propeller [7].
 Figure 3. Integrin Dimer Structure: Globular domain structures of α and β subunits in a stable
dimer. Ligand binding happens at the interface of the αI (left panel) or
β-propeller (right panel) and the βI domain.
ii) Dimerization
Figure 3. Integrin Dimer Structure: Globular domain structures of α and β subunits in a stable
dimer. Ligand binding happens at the interface of the αI (left panel) or
β-propeller (right panel) and the βI domain.
ii) Dimerization
Dimerization occurs via the β-propeller surface on the α chain and the hybrid domain in the β chain in the cytoplasm [8]. The sequences at these interacting surfaces seem to control the specificity of chain selection. The dimers have been shown to be stabilized and remain inactive by hydrophobic interactions and electrostatic salt bridges at the outer- and inner-membrane proximal regions respectively [9, 10].
iii) Interactions
The cytoplasmic tail of β-chain is known to bind to protein adaptors through NPxY/F motifs [11]; this activates the integrins by breaking the salt bridge between the dimer (reviewed in [12, 13]). In general, the adaptor proteins promote linkage to actin [14], however intermediate filaments have also been implicated via vimentin [15, 16].
Scaffolding adaptors (e.g. paxillin, kindlin) forms bridges between focal adhesion proteins
Catalytic adaptors (e.g. focal adhesion kinase, integrin-linked kinase, Src) propagate signal transduction from adhesion sites. Interactions via α-tail are not well established due to sequence variability, however, α-tail is implicated in the cell-type specific integrin activation through binding proteins that modulate downstream signaling [17, 18]. Phosphorylation state of cytoplasmic tail residues modulate the competition between adaptors for binding and hence the subsequent cytoskeletal interactions of integrins and response (reviewed in [19]).
The role of protein structure in ligand affinity modulation, signaling and dynamics of surface distribution of integrins is reviewed in[20].
Protein Structure
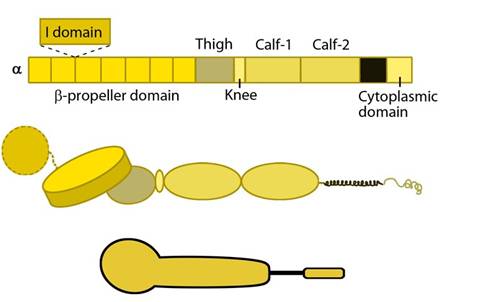 Figure 1. Integrin alpha chain: Integrin α subunit domains: Top: Linear domain arrangement. Middle: The globular structure formed by protein domains. Bottom: simplified version of the integrin α subunit. The αI domain is present in some subtypes of the α subunit.
Figure 1. Integrin alpha chain: Integrin α subunit domains: Top: Linear domain arrangement. Middle: The globular structure formed by protein domains. Bottom: simplified version of the integrin α subunit. The αI domain is present in some subtypes of the α subunit.The α subunit leg consists of a thigh and 2 calf domains that support the ligand binding head formed by a β-propeller domain with 7 repeats forming the blades (shown as a cylinder in the figure below). Some of the propeller blade domains contain calcium binding EF-hand domains on the lower side; these allosterically affect ligand binding [3]. An additional αI (interactive) domain containing ~200 residues is present in some vertebrate α chains [4] (nine human α subtypes) between the propeller repeats 2 and 3 [5]. This contains a metal-ion dependent adhesion site (MIDAS) which is important for ligand binding.
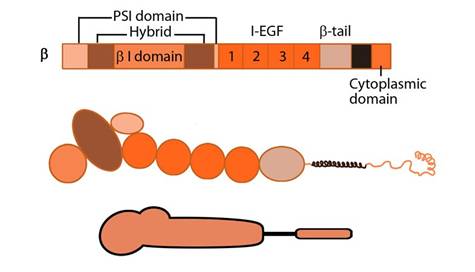 Figure 2. Integrin beta chain: Integrin β subunit domains: Top: Linear domain arrangement. Middle: The globular structure formed by protein domains. Bottom: simplified version of the integrin β subunit.
Figure 2. Integrin beta chain: Integrin β subunit domains: Top: Linear domain arrangement. Middle: The globular structure formed by protein domains. Bottom: simplified version of the integrin β subunit.i) Ligand binding
The βI domain binds ligand together with the β-propeller or with αI (if present) through MIDAS in a Mg2+ dependent fashion [6] at the interface in the headpiece. While Asp carboxyl group coordinates the βI MIDAS ion Mg2+, side chain hydrogen of the Arg of the RGD ligand binds directly to the Asp in domains 2 and 3 of β-propeller [7].
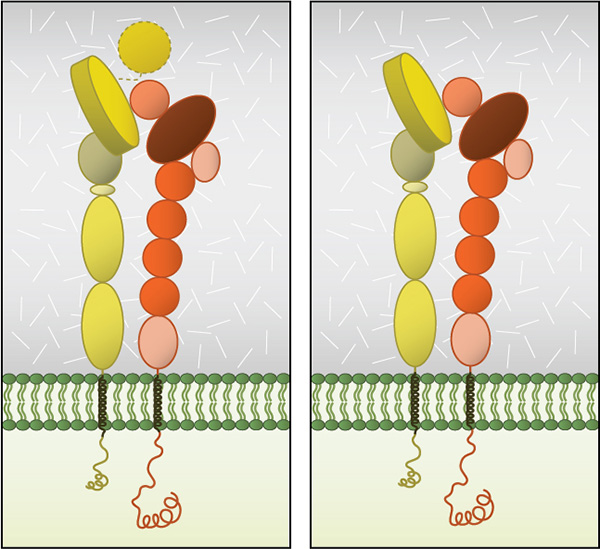 Figure 3. Integrin Dimer Structure: Globular domain structures of α and β subunits in a stable
dimer. Ligand binding happens at the interface of the αI (left panel) or
β-propeller (right panel) and the βI domain.
Figure 3. Integrin Dimer Structure: Globular domain structures of α and β subunits in a stable
dimer. Ligand binding happens at the interface of the αI (left panel) or
β-propeller (right panel) and the βI domain.Dimerization occurs via the β-propeller surface on the α chain and the hybrid domain in the β chain in the cytoplasm [8]. The sequences at these interacting surfaces seem to control the specificity of chain selection. The dimers have been shown to be stabilized and remain inactive by hydrophobic interactions and electrostatic salt bridges at the outer- and inner-membrane proximal regions respectively [9, 10].
iii) Interactions
The cytoplasmic tail of β-chain is known to bind to protein adaptors through NPxY/F motifs [11]; this activates the integrins by breaking the salt bridge between the dimer (reviewed in [12, 13]). In general, the adaptor proteins promote linkage to actin [14], however intermediate filaments have also been implicated via vimentin [15, 16].
Protein adaptors that bind to integrin cytoplasmic tails:
Structural adaptors (e.g. talin, filamin, tensin) link integrins directly to the cytoskeletonScaffolding adaptors (e.g. paxillin, kindlin) forms bridges between focal adhesion proteins
Catalytic adaptors (e.g. focal adhesion kinase, integrin-linked kinase, Src) propagate signal transduction from adhesion sites. Interactions via α-tail are not well established due to sequence variability, however, α-tail is implicated in the cell-type specific integrin activation through binding proteins that modulate downstream signaling [17, 18]. Phosphorylation state of cytoplasmic tail residues modulate the competition between adaptors for binding and hence the subsequent cytoskeletal interactions of integrins and response (reviewed in [19]).
The role of protein structure in ligand affinity modulation, signaling and dynamics of surface distribution of integrins is reviewed in[20].