Integrin functions[Edit]
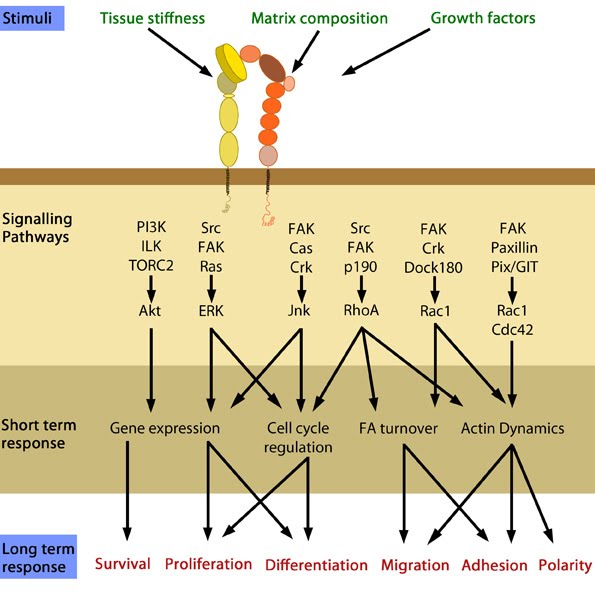 Figure 1. Cellular responses elicited by integrin signaling: In response to physical/chemical properties of the matrix and growth factors in the environment (outside-in signaling), integrins bind ligands and get activated. Accordingly, a variety of signaling pathways can be triggered mainly through the different kinases as mentioned above. These can bring about changes in one or more cellular events (short term responses) that eventually result in global (long term) responses in cellular behavior. Adapted from [1].
Figure 1. Cellular responses elicited by integrin signaling: In response to physical/chemical properties of the matrix and growth factors in the environment (outside-in signaling), integrins bind ligands and get activated. Accordingly, a variety of signaling pathways can be triggered mainly through the different kinases as mentioned above. These can bring about changes in one or more cellular events (short term responses) that eventually result in global (long term) responses in cellular behavior. Adapted from [1].1) Integrins undergo a process called activation, during which conformational changes expose the headpiece (βI and hybrid domain) for ligand binding [2, 3, 4, 5]. This can be initiated by the binding of adaptor proteins and/or ligands.
2) Adaptor proteins bind to the integrin cytoplasmic domains, thereby connecting integrin to the cytoskeleton.
3) Integrins microcluster laterally for efficient ligand binding.
Upon activation, integrins are capable of triggering a variety of signal transduction cascades. The combination of α and β subtypes, for example, will affect different in vivo functions. As demonstrated by knockout mouse studies, and highlighted in the table below, these include cell behaviour and tissue organization (reviewed in [6, 7, 8, 9]).
Which signalling pathway is initiated by integrin activation is based on the biological context, as well as the ligands bound (matrix components/growth factors). Depending on the combination of these factors, a variety of short-term and long-term responses may result [4]. Substrate stiffness has also been shown to affect the type of adhesion structure formed following integrin activation.
One study revealed the development of podosome-like adhesion structures in non-transformed fibroblasts grown on fluid, membrane based substrates. In this case, integrin was activated by membrane bound RGD (Arg-Gly-Asp) peptides. When grown on rigid surfaces, RGD-activated integrin would normally initiate the formation of focal adhesions [10]. The adhesion structures formed on the softer substrates had a similar morphology and makeup to classic podosomes found in macrophages. However, despite also being protrusive, the physiological function of these podosome-like structures remained unknown. The formation of these podosome-like structures in the absence of forces was mediated by p85beta recruitment and local PIP3 enrichment at the adhesion sites; both of which are not observed in focal adhesion formation. The increased production of PIP3 then caused N-WASP activation and RhoA-GAP ARAP3 recruitment, which downregulates RhoA-GTP level in podosome-forming cells.
2) Adaptor proteins bind to the integrin cytoplasmic domains, thereby connecting integrin to the cytoskeleton.
3) Integrins microcluster laterally for efficient ligand binding.
Upon activation, integrins are capable of triggering a variety of signal transduction cascades. The combination of α and β subtypes, for example, will affect different in vivo functions. As demonstrated by knockout mouse studies, and highlighted in the table below, these include cell behaviour and tissue organization (reviewed in [6, 7, 8, 9]).
Table: Role of some integrin subtypes in specific in vivo functions
| Integrin type |
In vivo function |
| β1 integrins |
Development |
| αV |
Vasculogenesis |
| α9β1 |
Lymphangiogenesis |
| αIIbβ3 | Thrombus formation |
| α6β4 | Integrity of skin |
| αVβ3 | Suppresses tumorigenesis, angiogenesis, wound healing, inflammation and atherosclerosis |
| β2 integrins | Immune responses |
Which signalling pathway is initiated by integrin activation is based on the biological context, as well as the ligands bound (matrix components/growth factors). Depending on the combination of these factors, a variety of short-term and long-term responses may result [4]. Substrate stiffness has also been shown to affect the type of adhesion structure formed following integrin activation.
One study revealed the development of podosome-like adhesion structures in non-transformed fibroblasts grown on fluid, membrane based substrates. In this case, integrin was activated by membrane bound RGD (Arg-Gly-Asp) peptides. When grown on rigid surfaces, RGD-activated integrin would normally initiate the formation of focal adhesions [10]. The adhesion structures formed on the softer substrates had a similar morphology and makeup to classic podosomes found in macrophages. However, despite also being protrusive, the physiological function of these podosome-like structures remained unknown. The formation of these podosome-like structures in the absence of forces was mediated by p85beta recruitment and local PIP3 enrichment at the adhesion sites; both of which are not observed in focal adhesion formation. The increased production of PIP3 then caused N-WASP activation and RhoA-GAP ARAP3 recruitment, which downregulates RhoA-GTP level in podosome-forming cells.