Activation of MMPs[Edit]
MMPs are produced as inactive precursors which are exposed to the extracellular milieu either via secretion or translocation to the membrane. Once exposed to the extracellular environment they are activated through proteolytic cleavage of the N terminal autoinhibitory domain.  Figure 1. Variations in MMP structure: This schematic diagram illustrates variations on the common MMP structure (reviewed in [1]). (A) MMP2 and MMP9 have additional fibronectin domain inserts within the catalytic domain (B)
Membrane bound MMPs (MT-MMPs) contain an additional membrane linkage
domain attached to the hemopexin domain, in the form of either a GPI
(glycosylphosphatidylinositol) anchor or a transmembrane domain. (C) Minimal MMPs, such as MMP7 and MMP26, do not contain a linker peptide or hemopexin domain.Cleavage is carried out by a range of different proteases including the MMPs themselves, as well as tissue proteases such as tryptase, plasmin and kallikrein [4]. Following activation, MMPs are able to digest various ECM components including a host of collagen types (I-V, VII, X, XI), gelatin, laminin, basement membrane proteins entactin and perlecan and ECM glycoproteins such as tenascin [4]. Active MMPs are involved in a wide range of cellular processes including; tissue remodeling, cell survival, immune cell migration and cancer pathology [3].
Figure 1. Variations in MMP structure: This schematic diagram illustrates variations on the common MMP structure (reviewed in [1]). (A) MMP2 and MMP9 have additional fibronectin domain inserts within the catalytic domain (B)
Membrane bound MMPs (MT-MMPs) contain an additional membrane linkage
domain attached to the hemopexin domain, in the form of either a GPI
(glycosylphosphatidylinositol) anchor or a transmembrane domain. (C) Minimal MMPs, such as MMP7 and MMP26, do not contain a linker peptide or hemopexin domain.Cleavage is carried out by a range of different proteases including the MMPs themselves, as well as tissue proteases such as tryptase, plasmin and kallikrein [4]. Following activation, MMPs are able to digest various ECM components including a host of collagen types (I-V, VII, X, XI), gelatin, laminin, basement membrane proteins entactin and perlecan and ECM glycoproteins such as tenascin [4]. Active MMPs are involved in a wide range of cellular processes including; tissue remodeling, cell survival, immune cell migration and cancer pathology [3].
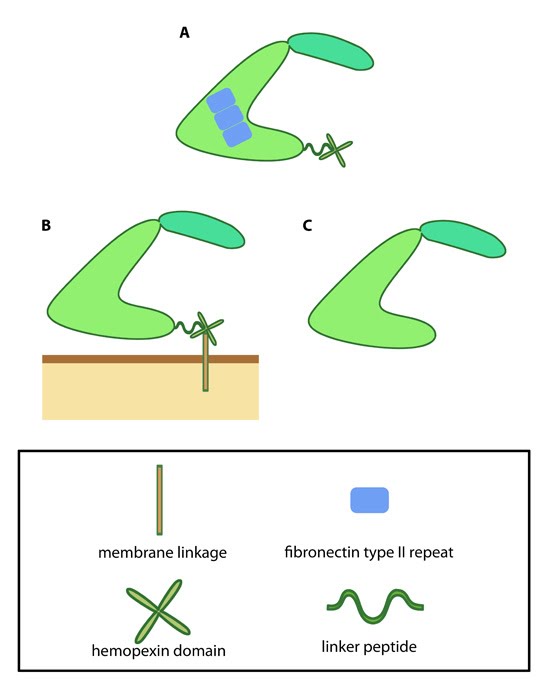 Figure 1. Variations in MMP structure: This schematic diagram illustrates variations on the common MMP structure (reviewed in [1]). (A) MMP2 and MMP9 have additional fibronectin domain inserts within the catalytic domain (B)
Membrane bound MMPs (MT-MMPs) contain an additional membrane linkage
domain attached to the hemopexin domain, in the form of either a GPI
(glycosylphosphatidylinositol) anchor or a transmembrane domain. (C) Minimal MMPs, such as MMP7 and MMP26, do not contain a linker peptide or hemopexin domain.
Figure 1. Variations in MMP structure: This schematic diagram illustrates variations on the common MMP structure (reviewed in [1]). (A) MMP2 and MMP9 have additional fibronectin domain inserts within the catalytic domain (B)
Membrane bound MMPs (MT-MMPs) contain an additional membrane linkage
domain attached to the hemopexin domain, in the form of either a GPI
(glycosylphosphatidylinositol) anchor or a transmembrane domain. (C) Minimal MMPs, such as MMP7 and MMP26, do not contain a linker peptide or hemopexin domain.