Nucleation Promoting Factors and NPF Accessory Proteins regulate actin filament polymerization[Edit]
Additional proteins are required for the initiation and regulation of actin filament polymerization. These include the Nucleation Promoting Factors (NPF) and NPF Accessory Proteins.
 Figure 1. WASP_WAVE_nucleation_promoting_factors: The molecular organization of Scar/WAVE family members and WASP, with examples of how the NPFs are represented in figures throughout this resource. Relevant domains believed to be important for binding to actin and for protein-protein interactions are highlighted (reviewed in [5, 6]). WHD= WAVE (also known as SCAR)-homology domain; WIP= WASP-interacting protein; V= verprolin-homology domain (i.e. WASP-homology-2 domain [WH2]); C= cofilin-homology domain; A= acidic-rich domain; WH1= WASP-homology-1 (aka Ena-VASP-homology-1 [EVH1]); GBD= GTPase binding domain. Of note: N-WASP contains an additional V domain, and the Scar/WAVE proteins lack a direct binding domain for the Rho GTPase family.Class I proteins are classified into five groups:
Figure 1. WASP_WAVE_nucleation_promoting_factors: The molecular organization of Scar/WAVE family members and WASP, with examples of how the NPFs are represented in figures throughout this resource. Relevant domains believed to be important for binding to actin and for protein-protein interactions are highlighted (reviewed in [5, 6]). WHD= WAVE (also known as SCAR)-homology domain; WIP= WASP-interacting protein; V= verprolin-homology domain (i.e. WASP-homology-2 domain [WH2]); C= cofilin-homology domain; A= acidic-rich domain; WH1= WASP-homology-1 (aka Ena-VASP-homology-1 [EVH1]); GBD= GTPase binding domain. Of note: N-WASP contains an additional V domain, and the Scar/WAVE proteins lack a direct binding domain for the Rho GTPase family.Class I proteins are classified into five groups:
1) Wiskott-Aldrich Syndrome protein (WASP) and neuronal-enriched homologue of WASP (N-WASP)
2) WASP family Verprolin-homologous (WAVE) proteins (aka Suppressor of cAMP receptor [Scar])
3) WASP and Scar homologue (WASH)
4) WASP homologue associated with actin, membranes and microtubules (WHAMM)
5) junction-mediating regulatory protein (JMY)
Class II includes proteins such as cortactin.
The conserved verpolin-cofilin-homology and acidic-rich (VCA) domain at the carboxy terminus of WASP and Scar family members binds directly to the Arp2/3 complex to increase its nucleation activity [7, 8, 9, 10]. WASp also associates with other signaling components (e.g. haemopoietic cell kinase) and formins to modulate actin polymerization for cell polarization and chemotaxis in neutrophils [11, 12]. WASp and formins also cooperate to control the balance between lamellipodial protrusion activity in epithelial cells [13]. The WASP family of NPFs are normally auto-inhibited due to protein interactions which prevent the NPF from associating with actin and the Arp2/3 complex; they are activated by Rho GTPases and PIP2 [8] (reviewed in [14, 15]). In contrast, the Scar/WAVE proteins have constitutive activity [16]. NPF accessory proteins also modulate the activity of NPFs.
Examples of NPF accessory proteins include Verprolin (yeast), which modulates the activity of WASp with type I myosins, to promote actin assembly by Arp2/3 complex [17]. WASp-interacting proteins (WIPs) will also regulate the WASp activity. For example WIP [18] not only inhibits N-WASP, but also promotes nucleation and activation of the Arp2/3 complex through the coordinated binding of actin and another NPF, cortactin [19]. SPIN90/WISH (SH3 protein interacting with Nck, 90 kDa/WASP-interacting SH3 protein) [20]) on the other hand increases actin assembly in dendritic filopodia/spines independently of N-WASP through its association with the neuron-specific scaffolding protein, PSD-95 [20]. Surprisingly, indirect evidence shows that WIPs are required for WASp function [21].
Nucleation Promoting Factors
NPFs (e.g. WASP, Scar/WAVE) modulate actin filament nucleation by bringing together actin monomers and pre-existing actin filaments, for example, during filopodial initiation where they recruit the Arp2/3 complex. NPFs compete with profilin for binding to free actin (which inhibits actin nucleotide exchange) [1,2, 3, 4]; these combined functions promote actin-filament assembly at the barbed end.
Mammalian NPFs are broadly grouped into 2 classes:
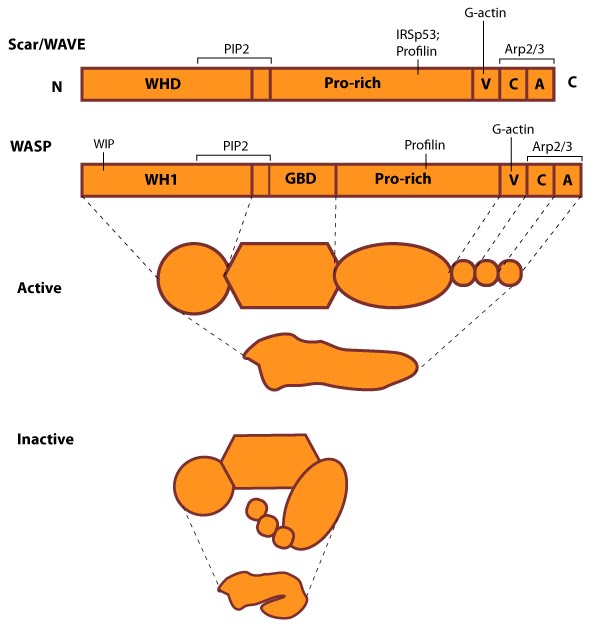 Figure 1. WASP_WAVE_nucleation_promoting_factors: The molecular organization of Scar/WAVE family members and WASP, with examples of how the NPFs are represented in figures throughout this resource. Relevant domains believed to be important for binding to actin and for protein-protein interactions are highlighted (reviewed in [5, 6]). WHD= WAVE (also known as SCAR)-homology domain; WIP= WASP-interacting protein; V= verprolin-homology domain (i.e. WASP-homology-2 domain [WH2]); C= cofilin-homology domain; A= acidic-rich domain; WH1= WASP-homology-1 (aka Ena-VASP-homology-1 [EVH1]); GBD= GTPase binding domain. Of note: N-WASP contains an additional V domain, and the Scar/WAVE proteins lack a direct binding domain for the Rho GTPase family.
Figure 1. WASP_WAVE_nucleation_promoting_factors: The molecular organization of Scar/WAVE family members and WASP, with examples of how the NPFs are represented in figures throughout this resource. Relevant domains believed to be important for binding to actin and for protein-protein interactions are highlighted (reviewed in [5, 6]). WHD= WAVE (also known as SCAR)-homology domain; WIP= WASP-interacting protein; V= verprolin-homology domain (i.e. WASP-homology-2 domain [WH2]); C= cofilin-homology domain; A= acidic-rich domain; WH1= WASP-homology-1 (aka Ena-VASP-homology-1 [EVH1]); GBD= GTPase binding domain. Of note: N-WASP contains an additional V domain, and the Scar/WAVE proteins lack a direct binding domain for the Rho GTPase family.1) Wiskott-Aldrich Syndrome protein (WASP) and neuronal-enriched homologue of WASP (N-WASP)
2) WASP family Verprolin-homologous (WAVE) proteins (aka Suppressor of cAMP receptor [Scar])
3) WASP and Scar homologue (WASH)
4) WASP homologue associated with actin, membranes and microtubules (WHAMM)
5) junction-mediating regulatory protein (JMY)
Class II includes proteins such as cortactin.
The conserved verpolin-cofilin-homology and acidic-rich (VCA) domain at the carboxy terminus of WASP and Scar family members binds directly to the Arp2/3 complex to increase its nucleation activity [7, 8, 9, 10]. WASp also associates with other signaling components (e.g. haemopoietic cell kinase) and formins to modulate actin polymerization for cell polarization and chemotaxis in neutrophils [11, 12]. WASp and formins also cooperate to control the balance between lamellipodial protrusion activity in epithelial cells [13]. The WASP family of NPFs are normally auto-inhibited due to protein interactions which prevent the NPF from associating with actin and the Arp2/3 complex; they are activated by Rho GTPases and PIP2 [8] (reviewed in [14, 15]). In contrast, the Scar/WAVE proteins have constitutive activity [16]. NPF accessory proteins also modulate the activity of NPFs.
NPF Accessory Proteins
Accessory proteins bind to WASp and Scar/WAVE proteins to modulate their NPF activity.Examples of NPF accessory proteins include Verprolin (yeast), which modulates the activity of WASp with type I myosins, to promote actin assembly by Arp2/3 complex [17]. WASp-interacting proteins (WIPs) will also regulate the WASp activity. For example WIP [18] not only inhibits N-WASP, but also promotes nucleation and activation of the Arp2/3 complex through the coordinated binding of actin and another NPF, cortactin [19]. SPIN90/WISH (SH3 protein interacting with Nck, 90 kDa/WASP-interacting SH3 protein) [20]) on the other hand increases actin assembly in dendritic filopodia/spines independently of N-WASP through its association with the neuron-specific scaffolding protein, PSD-95 [20]. Surprisingly, indirect evidence shows that WIPs are required for WASp function [21].
In another example, the WAVE complex will inhibit Scar/WAVE proteins from activating the Arp2/3 complex. The NPF activity of Scar/WAVE is restored during nucleation when Rac-GTP causes the dissociation of the WAVE complex from WAVE1 [22]. Similarly, IRSp53 has been implicated in both lamellipodia and filopodia formation/protrusion by augmenting the Rac-GTP-induced activation of WAVE NPF activity [23, 24].