Introduction to Actin Filament Depolymerization[Edit]
Whole cell motility and mechanosensing rely on the continual restructuring of the cytoskeleton, particularly within lamellipodia and filopodia; two dynamic structures that contribute to cell motility. Actin filament depolymerization ensures the turnover of actin filaments within these structures and maintains a pool of actin monomers that permits the continual restructuring and growth of the actin cytoskeleton.
 Figure 1. ADF/cofilin influences actin filament turnover: ADF/cofilin influences actin filament turnover. ADF cooperatively binds to F-actin to increase the steady state turn-over (e.g. treadmilling rate) of actin filaments and accumulation of ADF-ADP-G-actin, ADP-G-actin, and ATP-G-actin (via nucleotide exchange). ADF binds faster to actin filaments that have barbed end capping proteins (e.g. gelsolin) (B). The actin subunits depolymerizing from ADF-bound filaments are recycled to filaments lacking ADF, thus maintaining the pool of actin-filaments. At high ADF concentration, polymerization of ADP-actin at the pointed end is favored on capped-filaments (A); alternatively, nucleotide exchange increases the pool of ATP-actin for barbed end assembly of new or existing filaments (C).
Disassembly of actin filaments occurs at the pointed end of the filament and is driven by the ADF/cofilin (AC) family of proteins. Actin monomers intrinsically dissociate from the barbed end at a faster rate than they do from the pointed end [1]. This is counteracted by the binding of capping proteins or formins to the barbed end, creating a more stable filament. The action of cofilin at the pointed end serves to destabilize the filament and promote the release of ADP-actin monomers.
Figure 1. ADF/cofilin influences actin filament turnover: ADF/cofilin influences actin filament turnover. ADF cooperatively binds to F-actin to increase the steady state turn-over (e.g. treadmilling rate) of actin filaments and accumulation of ADF-ADP-G-actin, ADP-G-actin, and ATP-G-actin (via nucleotide exchange). ADF binds faster to actin filaments that have barbed end capping proteins (e.g. gelsolin) (B). The actin subunits depolymerizing from ADF-bound filaments are recycled to filaments lacking ADF, thus maintaining the pool of actin-filaments. At high ADF concentration, polymerization of ADP-actin at the pointed end is favored on capped-filaments (A); alternatively, nucleotide exchange increases the pool of ATP-actin for barbed end assembly of new or existing filaments (C).
Disassembly of actin filaments occurs at the pointed end of the filament and is driven by the ADF/cofilin (AC) family of proteins. Actin monomers intrinsically dissociate from the barbed end at a faster rate than they do from the pointed end [1]. This is counteracted by the binding of capping proteins or formins to the barbed end, creating a more stable filament. The action of cofilin at the pointed end serves to destabilize the filament and promote the release of ADP-actin monomers.
The conversion of ATP-F-actin to ADP-F-actin involves the hydrolysis of ATP and subsequent release of free inorganic phosphate (Pi) molecules. These free Pi molecules bind antagonistically to cofilin and as such cofilin binding to F-actin precedes Pi release [2]. Whilst Pi stabilizes the actin filament once bound, AC proteins destabilize the filament by inducing conformational change. This involves the production or stabilization of a twist within the filament or between monomers, creating a strain that leads to the loss of filament integrity and disassembly.
The conformational change induced by cofilin binding further promotes filament destabilization through increasing the rate of Pi release by approximately 10-fold [2]. The pH of the environment also affects the ability of cofilin to depolymerize actin filaments, with a higher pH favoring depolymerization due to the weaker binding of Pi in more alkali environments [3].
Recent data suggests that the efficacy of cofilin in filament severing is enhanced in actin bundles despite slower binding kinetics of cofilin to fascin-bundled actin filaments. This enhanced efficacy permits the the severing of bundled actin filaments at concentrations of cofilin that are inadequate to induce severing of free filaments. It was proposed that this is the result of cross-linkers such as fascin reducing the flexibility of the actin filaments and subsequently making them more vulnerable to the twisting effect of cofilin; as the filament as a whole is unable to provide slack for the sections under pressure [4].
The destabilized form of actin filaments, which has been compared to that observed in younger filaments [5], is more prone to filament severing. The higher affinity of ACs for ADP-F-actin relative to ATP-F-actin causes severing in the central regions of filaments where ADP-actin is enriched, though depolymerization at the pointed end also occurs [6].
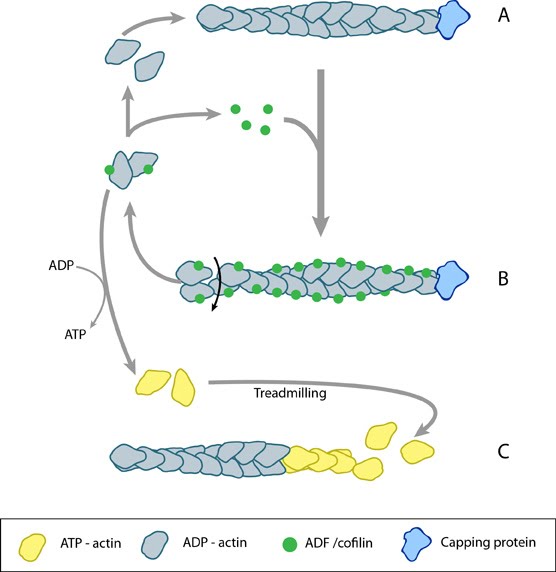 Figure 1. ADF/cofilin influences actin filament turnover: ADF/cofilin influences actin filament turnover. ADF cooperatively binds to F-actin to increase the steady state turn-over (e.g. treadmilling rate) of actin filaments and accumulation of ADF-ADP-G-actin, ADP-G-actin, and ATP-G-actin (via nucleotide exchange). ADF binds faster to actin filaments that have barbed end capping proteins (e.g. gelsolin) (B). The actin subunits depolymerizing from ADF-bound filaments are recycled to filaments lacking ADF, thus maintaining the pool of actin-filaments. At high ADF concentration, polymerization of ADP-actin at the pointed end is favored on capped-filaments (A); alternatively, nucleotide exchange increases the pool of ATP-actin for barbed end assembly of new or existing filaments (C).
Figure 1. ADF/cofilin influences actin filament turnover: ADF/cofilin influences actin filament turnover. ADF cooperatively binds to F-actin to increase the steady state turn-over (e.g. treadmilling rate) of actin filaments and accumulation of ADF-ADP-G-actin, ADP-G-actin, and ATP-G-actin (via nucleotide exchange). ADF binds faster to actin filaments that have barbed end capping proteins (e.g. gelsolin) (B). The actin subunits depolymerizing from ADF-bound filaments are recycled to filaments lacking ADF, thus maintaining the pool of actin-filaments. At high ADF concentration, polymerization of ADP-actin at the pointed end is favored on capped-filaments (A); alternatively, nucleotide exchange increases the pool of ATP-actin for barbed end assembly of new or existing filaments (C).The conversion of ATP-F-actin to ADP-F-actin involves the hydrolysis of ATP and subsequent release of free inorganic phosphate (Pi) molecules. These free Pi molecules bind antagonistically to cofilin and as such cofilin binding to F-actin precedes Pi release [2]. Whilst Pi stabilizes the actin filament once bound, AC proteins destabilize the filament by inducing conformational change. This involves the production or stabilization of a twist within the filament or between monomers, creating a strain that leads to the loss of filament integrity and disassembly.
The conformational change induced by cofilin binding further promotes filament destabilization through increasing the rate of Pi release by approximately 10-fold [2]. The pH of the environment also affects the ability of cofilin to depolymerize actin filaments, with a higher pH favoring depolymerization due to the weaker binding of Pi in more alkali environments [3].
Recent data suggests that the efficacy of cofilin in filament severing is enhanced in actin bundles despite slower binding kinetics of cofilin to fascin-bundled actin filaments. This enhanced efficacy permits the the severing of bundled actin filaments at concentrations of cofilin that are inadequate to induce severing of free filaments. It was proposed that this is the result of cross-linkers such as fascin reducing the flexibility of the actin filaments and subsequently making them more vulnerable to the twisting effect of cofilin; as the filament as a whole is unable to provide slack for the sections under pressure [4].
The destabilized form of actin filaments, which has been compared to that observed in younger filaments [5], is more prone to filament severing. The higher affinity of ACs for ADP-F-actin relative to ATP-F-actin causes severing in the central regions of filaments where ADP-actin is enriched, though depolymerization at the pointed end also occurs [6].