ADF / Cofilin in actin filament deploymerization[Edit]
The AC protein family includes actin depolymerizing factor (ADF [1] aka destrin (destroys F-actin)[2, 3], actophorin (found in Acanthamoeba castellanii [4] and depactin (found in starfish oocytes [5]). Both the phosphorylation of ACs [6] and the membrane lipids, phosphatidyl 4-phosphate (PIP) and 4,5-bis-phosphate (PIP2) [7], greatly reduce the F-actin binding and depolymerizing activity of ACs. AC phosphorylation in vertebrates is controlled by the activity of Rho GTPase and Lim kinase pathways [8, 9]. ADF/cofilin contribute to actin dynamics in a number of cell structures including the lamellipodium, filopodium, the postsynaptic density in dendritic spines [10] and invadopodia [11].
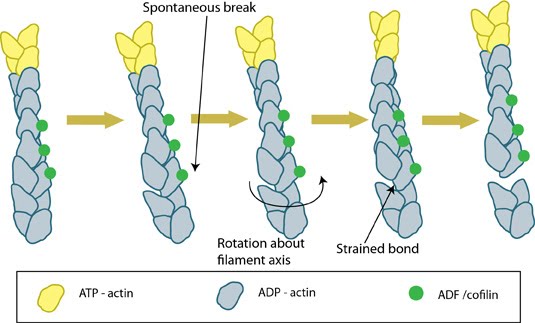
Mechanistically, cofilin binds between actin subunits when a longitudinal bond spontaneously breaks as the filament bends in thermal motion [11]. Cooperative binding of ADF/cofilin causes the filament to twist and structurally weaken [12]; this causes a modest severing effect that results in pointed end depolymerization and a 2-3 fold decrease in the average length [13, 14]. ACs promote shortening of actin filaments in two main ways: by severing the filament to create more ends that disassemble [15, 16, 17] and by promoting subunit loss from filament ends [13, 18, 19].
ACs promote filament turnover by altering the kinetics of filament disassembly. Recent findings have found that cofilin is more efficacious at severing actin filaments that are are cross-linked in actin bundles [20]. This is believed to result from the filament being more susceptible to the cofilin-induced twist, as its rigidity is maintained by the cross-linkers and it is unable flex under the added tension.
ACs bind both F and G actin-ADP with greater avidity than they bind to F or G actin-ATP and ADF-actin-ADP has a higher dissociation rate than actin-ADP [13, 16]. ACs also promote debranching by accelerating the rate-limiting release of the γ-phosphate from ADP-Pi-actin filament subunits [21]. Lastly, ACs interact with the Arp2/3 complex and ADP-actin subunits at the free pointed ends to aid in disassembly [22].
Interestingly, the nucleation of ADP-actin is greatly facilitated by high ADF concentrations; the ADF-ADP-G-actin complex is stabilzed, the treadmilling rate declines, and pointed-end assembly of ADP-G-actin is favored due to lateral stability of actin-filaments [23]. Increased lateral stability of the filaments may form a selection process that stabilizes filaments in bundles and may account for the emergence and extension of actin-based structures such as filopodia [24]. Further support for the role of ADF/cofilin in the stabilization of actin filaments comes from invadopodia, where the loss of cofilin results in a decrease in invadopodia lifetime [25].
Mechanistically, cofilin binds between actin subunits when a longitudinal bond spontaneously breaks as the filament bends in thermal motion [11]. Cooperative binding of ADF/cofilin causes the filament to twist and structurally weaken [12]; this causes a modest severing effect that results in pointed end depolymerization and a 2-3 fold decrease in the average length [13, 14]. ACs promote shortening of actin filaments in two main ways: by severing the filament to create more ends that disassemble [15, 16, 17] and by promoting subunit loss from filament ends [13, 18, 19].
ACs promote filament turnover by altering the kinetics of filament disassembly. Recent findings have found that cofilin is more efficacious at severing actin filaments that are are cross-linked in actin bundles [20]. This is believed to result from the filament being more susceptible to the cofilin-induced twist, as its rigidity is maintained by the cross-linkers and it is unable flex under the added tension.
ACs bind both F and G actin-ADP with greater avidity than they bind to F or G actin-ATP and ADF-actin-ADP has a higher dissociation rate than actin-ADP [13, 16]. ACs also promote debranching by accelerating the rate-limiting release of the γ-phosphate from ADP-Pi-actin filament subunits [21]. Lastly, ACs interact with the Arp2/3 complex and ADP-actin subunits at the free pointed ends to aid in disassembly [22].
Interestingly, the nucleation of ADP-actin is greatly facilitated by high ADF concentrations; the ADF-ADP-G-actin complex is stabilzed, the treadmilling rate declines, and pointed-end assembly of ADP-G-actin is favored due to lateral stability of actin-filaments [23]. Increased lateral stability of the filaments may form a selection process that stabilizes filaments in bundles and may account for the emergence and extension of actin-based structures such as filopodia [24]. Further support for the role of ADF/cofilin in the stabilization of actin filaments comes from invadopodia, where the loss of cofilin results in a decrease in invadopodia lifetime [25].
Differences between ADF and Cofilin
It should be noted that the AC proteins have been shown to initiate nucleation of new filaments from recently disassembled monomers. In this case, cofilin shows a higher nucleating activity (double) compared to ADF [3]. Based on this finding, which was observed at pH8 in experimental conditions, ADF is considered to be more effective in promoting actin turnover as the free monomers are less likely to undergo ADF mediated nucleation immediately following disassembly [3].
It should be noted that the AC proteins have been shown to initiate nucleation of new filaments from recently disassembled monomers. In this case, cofilin shows a higher nucleating activity (double) compared to ADF [3]. Based on this finding, which was observed at pH8 in experimental conditions, ADF is considered to be more effective in promoting actin turnover as the free monomers are less likely to undergo ADF mediated nucleation immediately following disassembly [3].