Myosin-X transport cargo along actin filaments[Edit]
Specific members of the Myosin superfamily of motor proteins are known to transport cargo along actin filaments. Myosin-V and Myosin-X are two examples. Although the specifics of their movement are distinct, the concept is the same, and can be understood when considering how Myosin-X transports components down the length of a growing filopodia.
The transport of components along filopodial shafts is crucial to the continued growth of actin filaments and the formation of adhesions at the tips of filopodia. Myosin-X facilitates the transport of components from the cell body to filopodial tips, resulting in their integration into the cell membrane as receptors/adhesions or their use in the growth of actin filaments and filopodia [1, 2, 3, 4, 5].
 Figure 1. Myosin-X step size: Myosin-X step size corresponds to a single twist of the actin filament helix. Binding sites are represented by red and dark blue actin monomers. In this figure we see how Myosin-X is able to step forwards and backwards (A) as well as horizontally, between filaments (B)
Movement of myosin-X is driven by ATP hydrolysis, in a unique mechanism that resembles walking or stepping. This movement is known to occur preferentially on actin bundles rather than single actin filaments [6, 7]. Although it is essentially a forward movement, evidence indicates that the protein may also take side-steps. This may be carried out as a means of overcoming obstacles or defects in the track [7].
Figure 1. Myosin-X step size: Myosin-X step size corresponds to a single twist of the actin filament helix. Binding sites are represented by red and dark blue actin monomers. In this figure we see how Myosin-X is able to step forwards and backwards (A) as well as horizontally, between filaments (B)
Movement of myosin-X is driven by ATP hydrolysis, in a unique mechanism that resembles walking or stepping. This movement is known to occur preferentially on actin bundles rather than single actin filaments [6, 7]. Although it is essentially a forward movement, evidence indicates that the protein may also take side-steps. This may be carried out as a means of overcoming obstacles or defects in the track [7].
The walking mechanism of myosin-X is known to be distinct from other walking myosins such as myosin-V;. This is particularly relevant to the step size and the preference for specific actin bundling proteins [7]. Whilst myosin-V takes steps of 36nm and follows single filaments rather than side-stepping along parallel filaments of the bundle, myosin-X takes shorter steps which have been measured at 17.5nm [6, 7]. This shorter stepping distance may explain why myosin-X uses binding sites on parallel filaments, as a short step on a single filament would result in the protein rotating around the natural twist of the filament [7].
A number of components essential to the growth and function of filopodia are carried by myosin-X, including β-integrin [3]. β-integrin is essential for focal adhesion formation and binds to myosin-X via the FERM domain. Knock down of myosin-X by siRNA was shown to disrupt the formation of adhesions to collagen I, particularly at early time points, with no disruption after 60 minutes. This highlights the importance of myosin-X in the initial stages of integrin-mediated adhesion formation [3].
Other cargo transported by myosin-X includes neogenin and ‘deleted in colorectal cancer’ (DCC), which function as netrin receptors. In addition, DCC anchors translational machinery such a ribosomes to the membranes of neuronal growth cones and dendrites [8, 4].
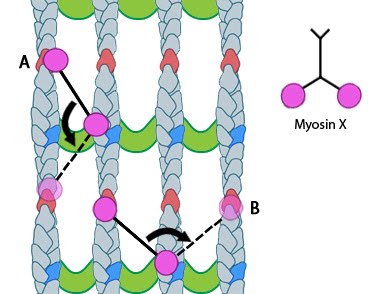 Figure 1. Myosin-X step size: Myosin-X step size corresponds to a single twist of the actin filament helix. Binding sites are represented by red and dark blue actin monomers. In this figure we see how Myosin-X is able to step forwards and backwards (A) as well as horizontally, between filaments (B)
Figure 1. Myosin-X step size: Myosin-X step size corresponds to a single twist of the actin filament helix. Binding sites are represented by red and dark blue actin monomers. In this figure we see how Myosin-X is able to step forwards and backwards (A) as well as horizontally, between filaments (B)The walking mechanism of myosin-X is known to be distinct from other walking myosins such as myosin-V;. This is particularly relevant to the step size and the preference for specific actin bundling proteins [7]. Whilst myosin-V takes steps of 36nm and follows single filaments rather than side-stepping along parallel filaments of the bundle, myosin-X takes shorter steps which have been measured at 17.5nm [6, 7]. This shorter stepping distance may explain why myosin-X uses binding sites on parallel filaments, as a short step on a single filament would result in the protein rotating around the natural twist of the filament [7].
Cargo Binding
Myosin-X is able to carry a variety of cargo to the tips of filopodia [1, 2, 3, 4]. Although the exact mechanism by which myosin-X selects cargo remains unclear it is well established that cargo recognition and binding results from the presence of the myosin tail homology 4 (MyTH4) and ezrin/radixin/moesin (FERM) domains – together these are known as the MyTH4-FERM cassette [8].A number of components essential to the growth and function of filopodia are carried by myosin-X, including β-integrin [3]. β-integrin is essential for focal adhesion formation and binds to myosin-X via the FERM domain. Knock down of myosin-X by siRNA was shown to disrupt the formation of adhesions to collagen I, particularly at early time points, with no disruption after 60 minutes. This highlights the importance of myosin-X in the initial stages of integrin-mediated adhesion formation [3].
Other cargo transported by myosin-X includes neogenin and ‘deleted in colorectal cancer’ (DCC), which function as netrin receptors. In addition, DCC anchors translational machinery such a ribosomes to the membranes of neuronal growth cones and dendrites [8, 4].