Formin mediates actin nucleation of unbranched filaments[Edit]
The formins are a large family of proteins that facilitate the nucleation of new, unbranched filaments by promoting the interaction between two actin monomers (reviewed in [1]). Under normal circumstances formins are auto-inhibited through structural interactions between the two ends of the protein [2]. However, conformational rearrangements resulting in their activation can be induced through interactions with GTP-bound (active) Rho GTPases [3]. This process remains poorly understood (reviewed in [4]).
 Figure 1. Formin-mediated nucleation of actin filaments: The FH2 domains of the formin dimer (shown in green)
bind to actin monomers to initiate filament assembly. Recent studies
indicate this is assisted, or even mediated, by additional factors such
as APC. The FH1 domains
of the formin dimer (shown as black lines) have short polyproline
sequences that interact with profilin. Profilin binds to both formin and
actin monomers to increase the addition of actin monomers to the barbed
end of the filament.In a recent study, the tumor suppressor adenomatous polyposis coli (APC) was shown to bind the formin mDia1 and overcome capping protein- and profilin-mediated suppression of spontaneous actin nucleation, resulting in the initiation of actin filament nucleation and elongation [5]. In the mechanism described, APC is primarily responsible for actin monomer recruitment, whilst mDia1 catalyzes filament elongation. Actin recruitment by APC did not involve capturing F-actin intermediates that had spontaneously formed nor did APC contribute to filament elongation. In this model, once actin polymerization commences the APC-mDia1 complex separates – mDia1 is propelled away from APC along with the growing barbed end of the filament and APC remains attached to the filament at the site of nucleation [5].
Figure 1. Formin-mediated nucleation of actin filaments: The FH2 domains of the formin dimer (shown in green)
bind to actin monomers to initiate filament assembly. Recent studies
indicate this is assisted, or even mediated, by additional factors such
as APC. The FH1 domains
of the formin dimer (shown as black lines) have short polyproline
sequences that interact with profilin. Profilin binds to both formin and
actin monomers to increase the addition of actin monomers to the barbed
end of the filament.In a recent study, the tumor suppressor adenomatous polyposis coli (APC) was shown to bind the formin mDia1 and overcome capping protein- and profilin-mediated suppression of spontaneous actin nucleation, resulting in the initiation of actin filament nucleation and elongation [5]. In the mechanism described, APC is primarily responsible for actin monomer recruitment, whilst mDia1 catalyzes filament elongation. Actin recruitment by APC did not involve capturing F-actin intermediates that had spontaneously formed nor did APC contribute to filament elongation. In this model, once actin polymerization commences the APC-mDia1 complex separates – mDia1 is propelled away from APC along with the growing barbed end of the filament and APC remains attached to the filament at the site of nucleation [5].
Although a consensus has yet to be reached for the mechanism of formin-mediated nucleation, it is now well-established that activated formins function as dimers and form a donut-shaped complex around terminal actin subunits, orientating themselves toward the (+) end of the actin filament or nucleus [6]. This binding is facilitated by FH2 (formin homology 2) domains within the formin monomers. Next, each formin monomer binds and captures profilin units, which are themselves already bound to G-actin monomers. This interaction is mediated by multiple stretches of polyproline residues within the FH1 domain of formins [7]. This domain is known to range from 15-229 residues, consist of between 35% and 100% proline residues, and contain up to 16 profilin binding sites [8]. Profilin maintains a steady pool of actin monomers by promoting ADP to ATP nucleotide exchange on G-actin[6]. These monomers of ATP-G-actin are then added the growing actin filament. The coupling of formin with the growing end prevents capping and allows continued growth of the filaments [9].
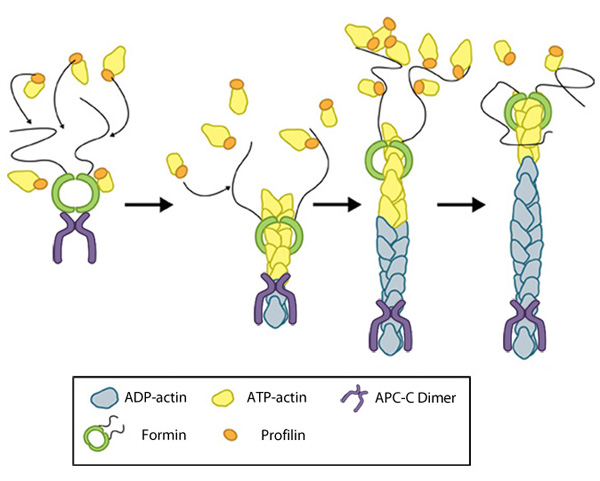 Figure 1. Formin-mediated nucleation of actin filaments: The FH2 domains of the formin dimer (shown in green)
bind to actin monomers to initiate filament assembly. Recent studies
indicate this is assisted, or even mediated, by additional factors such
as APC. The FH1 domains
of the formin dimer (shown as black lines) have short polyproline
sequences that interact with profilin. Profilin binds to both formin and
actin monomers to increase the addition of actin monomers to the barbed
end of the filament.
Figure 1. Formin-mediated nucleation of actin filaments: The FH2 domains of the formin dimer (shown in green)
bind to actin monomers to initiate filament assembly. Recent studies
indicate this is assisted, or even mediated, by additional factors such
as APC. The FH1 domains
of the formin dimer (shown as black lines) have short polyproline
sequences that interact with profilin. Profilin binds to both formin and
actin monomers to increase the addition of actin monomers to the barbed
end of the filament.Although a consensus has yet to be reached for the mechanism of formin-mediated nucleation, it is now well-established that activated formins function as dimers and form a donut-shaped complex around terminal actin subunits, orientating themselves toward the (+) end of the actin filament or nucleus [6]. This binding is facilitated by FH2 (formin homology 2) domains within the formin monomers. Next, each formin monomer binds and captures profilin units, which are themselves already bound to G-actin monomers. This interaction is mediated by multiple stretches of polyproline residues within the FH1 domain of formins [7]. This domain is known to range from 15-229 residues, consist of between 35% and 100% proline residues, and contain up to 16 profilin binding sites [8]. Profilin maintains a steady pool of actin monomers by promoting ADP to ATP nucleotide exchange on G-actin[6]. These monomers of ATP-G-actin are then added the growing actin filament. The coupling of formin with the growing end prevents capping and allows continued growth of the filaments [9].