Arp2/3-mediated nucleation of branched filaments[Edit]
The Arp2/3 complex is composed of 7 evolutionarily conserved subunits (Arp2, Arp3, ARPC1-C5) that are structurally similar to the barbed end of actin [1]. The complex is inherently inactive, however once activated it facilitates the nucleation of actin monomers from existing filaments as new branches or daughter filaments (reviewed in [2]).
Video: Arp2/3-mediated Actin Nucleation. The process by which the Arp2/3 complex mediates actin nucleation is shown here. This process is regulated by proteins such as WASp and Cdc42, which are depicted in this animation as stylized schematic models. [Video produced by MBInfo]
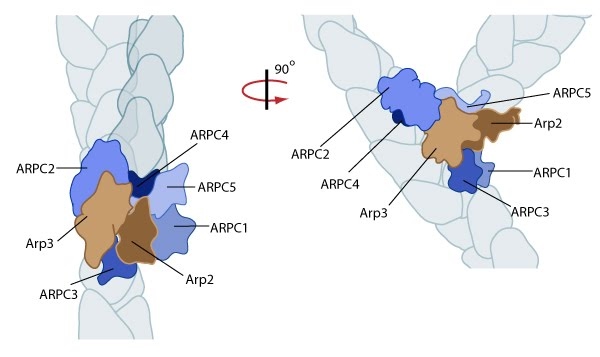 Figure 1. Reconstruction of Arp2/3 complex-mediated nucleation: Arp2/3 complex initiates a new branched filament by binding to the side of the mother filament and recruiting actin monomers. Components of the Arp2/3 complex remain at the pointed end of the filament (Figure adapted from [6]).
Figure 1. Reconstruction of Arp2/3 complex-mediated nucleation: Arp2/3 complex initiates a new branched filament by binding to the side of the mother filament and recruiting actin monomers. Components of the Arp2/3 complex remain at the pointed end of the filament (Figure adapted from [6]).The exact mechanism of nucleation remains unclear however it is believed that all seven subunits coordinate to anchor the pointed end of the new filament at a 78° angle to the existing actin network [6]. NPFs act to deliver actin subunits to the Arp2/3 complex at the barbed end, via binding through their WH2 domain – this serves to push the leading edge of a cell forward [11]. It has also been suggested that each component of the complex plays specific roles, with Arp2 and Arp3 interacting with the pointed end of the daughter filament and ARPC2 and ARPC4 binding to the original filament [6].
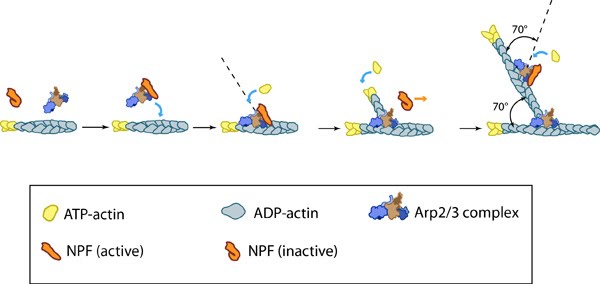 Figure 2. Arp2/3-mediated actin polymerization: NPFs (e.g. WASp; Scar) bring together the Arp2/3 complex and actin monomers to nucleate actin filaments that form new branches from the side of preexisting filaments. The Arp2/3 complex remains at the minus end of the filament.
Figure 2. Arp2/3-mediated actin polymerization: NPFs (e.g. WASp; Scar) bring together the Arp2/3 complex and actin monomers to nucleate actin filaments that form new branches from the side of preexisting filaments. The Arp2/3 complex remains at the minus end of the filament.This bias of Arp2/3 towards branch formation on convex curvature was proposed to represent a mechanosensing mechanism whereby detection of filament curvature alters the local cytoskeleton in order to reinforce it against the imposing forces [12].