Filamin[Edit]
The filamin family of proteins bind to both actin and a number of signaling molecules including Rho GTPases. Evidence for this was shown with the loss of Filamin-A in M2 Melanoma cells, which prevented RalA- and Cdc42-mediated filopodia formation [1]. Filamin A is necessary for the successful production of lamellipodia [2, 3], specifically due to its F-actin crosslinking activity [3]. Following recruitment to the membrane at the leading edge of a lamellipodium, it crosslinks newly polymerized actin filaments to enhance lamellipodium formation [3].
 Figure 1. Filamin: This schematic diagram illustrates the molecular organization of filamin [4] and provides examples for how the filamin dimer is represented in figures throughout this resource. The ABD is at the amino (N)-terminus in contrast to the opposite end (the carboxy [C]-terminus), which contains a significant number of protein-protein interaction domains (reviewed in [5]).Filamin is an actin-binding protein that was first recognized (and named) for its filamentous colocalization with actin stress fibers [6]. Filamin molecules are naturally found in cells as elongated, V-shaped dimers (i.e. two linked filamin molecules) [7] that contain several immunoglobulin-like domains for protein interactions, amino-terminal actin-binding domains (ABD) composed of two calponin homology (CH) domains, and a carboxy-terminal dimerization domain (see Figure below) [8] (reviewed in [1]).
Figure 1. Filamin: This schematic diagram illustrates the molecular organization of filamin [4] and provides examples for how the filamin dimer is represented in figures throughout this resource. The ABD is at the amino (N)-terminus in contrast to the opposite end (the carboxy [C]-terminus), which contains a significant number of protein-protein interaction domains (reviewed in [5]).Filamin is an actin-binding protein that was first recognized (and named) for its filamentous colocalization with actin stress fibers [6]. Filamin molecules are naturally found in cells as elongated, V-shaped dimers (i.e. two linked filamin molecules) [7] that contain several immunoglobulin-like domains for protein interactions, amino-terminal actin-binding domains (ABD) composed of two calponin homology (CH) domains, and a carboxy-terminal dimerization domain (see Figure below) [8] (reviewed in [1]).
Filamin binds all actin isoforms (e.g. F-actin, G-actin) and its structural organization allows it to form a flexible bridge between two actin filaments at various angles, thereby imparting the actin network with loose or gel-like qualities [9]. Filamin also simultaneously interacts with and influences the activity of a number of other diverse proteins (e.g. transmembrane receptors, cell adhesion molecules, signaling molecules) through its immunoglobulin-like domains (reviewed in [1, 10, 5]). In some instances, the majority of these repeats are utilized for protein-protein interactions (e.g. beta-integrin binding [11]).
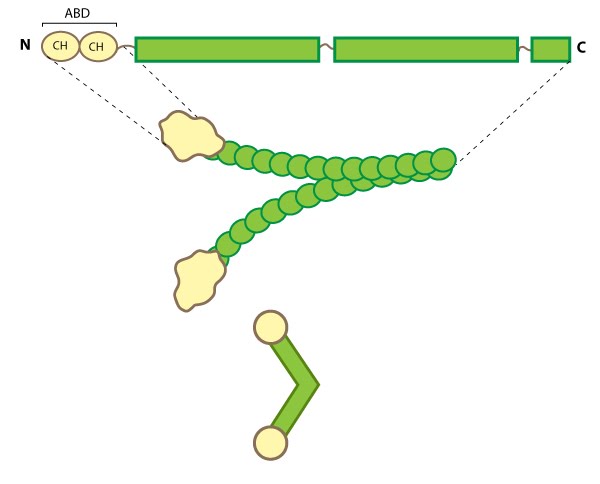 Figure 1. Filamin: This schematic diagram illustrates the molecular organization of filamin [4] and provides examples for how the filamin dimer is represented in figures throughout this resource. The ABD is at the amino (N)-terminus in contrast to the opposite end (the carboxy [C]-terminus), which contains a significant number of protein-protein interaction domains (reviewed in [5]).
Figure 1. Filamin: This schematic diagram illustrates the molecular organization of filamin [4] and provides examples for how the filamin dimer is represented in figures throughout this resource. The ABD is at the amino (N)-terminus in contrast to the opposite end (the carboxy [C]-terminus), which contains a significant number of protein-protein interaction domains (reviewed in [5]).Filamin binds all actin isoforms (e.g. F-actin, G-actin) and its structural organization allows it to form a flexible bridge between two actin filaments at various angles, thereby imparting the actin network with loose or gel-like qualities [9]. Filamin also simultaneously interacts with and influences the activity of a number of other diverse proteins (e.g. transmembrane receptors, cell adhesion molecules, signaling molecules) through its immunoglobulin-like domains (reviewed in [1, 10, 5]). In some instances, the majority of these repeats are utilized for protein-protein interactions (e.g. beta-integrin binding [11]).
Filamin Localization and Function
Filamin forms a vital scaffolding adaptor and regulatory component that contributes to the mechanical stability of cells by linking the internal actin network with membrane receptors and mechanosensitive components. This function correlates with its distribution in cultured cells along actin stress fibers, within cortical actin networks and sometimes at membrane ruffles [12, 13].
Numerous filamin isoforms exist in metazoan cells (reviewed in [1, 10]) and in some cases their cellular distribution suggests that certain isoforms may have specialized roles within the cell [14]. In muscle cells, filamin is locally concentrated at specific points called Z-lines, but in non-muscle cells filamin generally remains distributed throughout larger actin-based structures such as the lamellipodium, cortical actin, stress fibers and at sites of adhesion [reviewed in (15)].
Filamin not only binds to membrane receptors and to the Rho family of GTPases to influence actin organization (reviewed in [10]), but can itself directly transduce mechanical signals (e.g. stress) to regulate the cell stiffness [16] and possibly alter cellular adhesion [17]. Filamin can be phosphorylated by numerous kinases including cAMP-dependent protein kinase (i.e. protein kinase A) [18, 19] and protein kinase C [20]. Filamin phosphorylation not only regulates its interaction with other proteins and affects its ability to cross-link actin, but also regulates its stability and proteolysis (destruction) (reviewed in [1, 10]).
Numerous filamin isoforms exist in metazoan cells (reviewed in [1, 10]) and in some cases their cellular distribution suggests that certain isoforms may have specialized roles within the cell [14]. In muscle cells, filamin is locally concentrated at specific points called Z-lines, but in non-muscle cells filamin generally remains distributed throughout larger actin-based structures such as the lamellipodium, cortical actin, stress fibers and at sites of adhesion [reviewed in (15)].
Filamin not only binds to membrane receptors and to the Rho family of GTPases to influence actin organization (reviewed in [10]), but can itself directly transduce mechanical signals (e.g. stress) to regulate the cell stiffness [16] and possibly alter cellular adhesion [17]. Filamin can be phosphorylated by numerous kinases including cAMP-dependent protein kinase (i.e. protein kinase A) [18, 19] and protein kinase C [20]. Filamin phosphorylation not only regulates its interaction with other proteins and affects its ability to cross-link actin, but also regulates its stability and proteolysis (destruction) (reviewed in [1, 10]).