Fimbrin[Edit]
Fimbrin (aka plastin homologue, accumentin) is an actin binding protein that was originally identified in microvilli [1, 2].  Figure 1. Fimbrin: This
schematic diagram illustrates the molecular organization of fimbrin and
highlights the relevant domains for binding to actin filaments.Fimbrin represents one of the most basic structures of an actin crosslinking protein; it contains a calcium-binding domain consisting of a pair of EF hand domains, and a pair of actin binding domains (ABDs) composed of two calponin homology (CH) domains (see figure below). However no calcium sensitivity has been demonstrated for the EF domains. Fimbrin is found in a number of organisms from yeast to humans and those organisms containing multiple isoforms (e.g. humans have three) frequently exhibit isoform expression that is cell type or tissue specific (reviewed in [3, 4]).
Figure 1. Fimbrin: This
schematic diagram illustrates the molecular organization of fimbrin and
highlights the relevant domains for binding to actin filaments.Fimbrin represents one of the most basic structures of an actin crosslinking protein; it contains a calcium-binding domain consisting of a pair of EF hand domains, and a pair of actin binding domains (ABDs) composed of two calponin homology (CH) domains (see figure below). However no calcium sensitivity has been demonstrated for the EF domains. Fimbrin is found in a number of organisms from yeast to humans and those organisms containing multiple isoforms (e.g. humans have three) frequently exhibit isoform expression that is cell type or tissue specific (reviewed in [3, 4]).
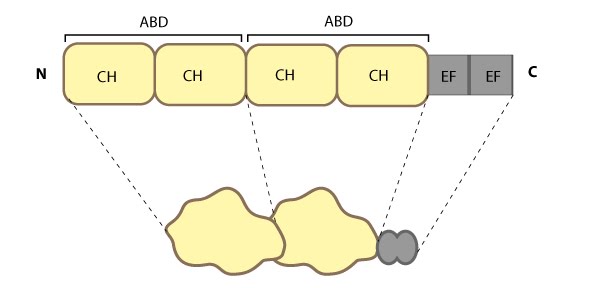 Figure 1. Fimbrin: This
schematic diagram illustrates the molecular organization of fimbrin and
highlights the relevant domains for binding to actin filaments.
Figure 1. Fimbrin: This
schematic diagram illustrates the molecular organization of fimbrin and
highlights the relevant domains for binding to actin filaments.Localization and function
Fimbrin primarily modulates the cytoskeleton organization of microvilli and stress fibers. Although fimbrin has been at the ends of stress fibers [5, 6][7]. Fimbrin is not only dispersed throughout the lamellipodium and lamella, but it is also found in various larger actin-based structures such as stereocilia [8][9, 10], cell adhesion sites, microspikes and membrane ruffles [2, 11][9] and it’s small size allows it to crosslink actin into rigid bundles.