Microfilament motor activity
Content
An introduction to actomyosin[Edit]
Actin filament networks, both within filopodia and lamellipodia, are highly dynamic structures. This characteristic is exemplified in the retrograde motion that is intrinsic to the ‘treadmilling’ mechanism of filament formation. Further to this motion, a number of cellular processes such as filopodial retraction and lamellipodial/lamellal contractions, rely on the rearward movement of the whole filament network or large filament bundles. The retrograde motion of actin treadmilling may play a minor role in aiding these processes, however additional factors are required.
One class of proteins that has been implicated in the translocation of F-actin is the myosin motor protein family. It remains unclear which isoforms contribute to this process in specific situations and to what extent [1, 2, 3, 4]. Each member of the myosin family possesses unique structural and functional properties, such as their step size, that determines their ability to engage in F-actin translocation [5]. It has been shown that myosins in general are required for this process to facilitate filopodial retraction [2].
Myosin II specifically, has been associated with F-actin retraction in several cell types including neurons [1], fibroblasts [6] and keratocytes [7], with particular emphasis on its role in the lamella and lamellipodia. Initially Myosin II was believed to influence F-actin dynamics and motility from within the lamella, as it had not been observed at the leading edge however it was recently observed within lamellipodia as protrusion reaches its peak, just prior to retraction [8]. This study postulates that myosin II is responsible for the formation and retrograde movement of actin arcs – bundles that form parallel to the leading edge and possibly contact multiple focal adhesions. This movement originates at the lamellipodium and moves rearward into the lamellum, producing a single continuously flowing actin network in the form of arcs [8]. It was consequently proposed that the rate of retrograde movement is reduced as the arcs contact focal adhesions near and within the lamella [8].
One class of proteins that has been implicated in the translocation of F-actin is the myosin motor protein family. It remains unclear which isoforms contribute to this process in specific situations and to what extent [1, 2, 3, 4]. Each member of the myosin family possesses unique structural and functional properties, such as their step size, that determines their ability to engage in F-actin translocation [5]. It has been shown that myosins in general are required for this process to facilitate filopodial retraction [2].
Myosin II specifically, has been associated with F-actin retraction in several cell types including neurons [1], fibroblasts [6] and keratocytes [7], with particular emphasis on its role in the lamella and lamellipodia. Initially Myosin II was believed to influence F-actin dynamics and motility from within the lamella, as it had not been observed at the leading edge however it was recently observed within lamellipodia as protrusion reaches its peak, just prior to retraction [8]. This study postulates that myosin II is responsible for the formation and retrograde movement of actin arcs – bundles that form parallel to the leading edge and possibly contact multiple focal adhesions. This movement originates at the lamellipodium and moves rearward into the lamellum, producing a single continuously flowing actin network in the form of arcs [8]. It was consequently proposed that the rate of retrograde movement is reduced as the arcs contact focal adhesions near and within the lamella [8].
Sarcomeres facilitate contractility of skeletal muscle[Edit]
A Role in Contraction Numerous motor proteins exist and each possess unique characteristics that allow them to facilitate different processes and functions. Even within the myosin superfamily variation exists in structure and function of each member. Not only can motor proteins translocate along microfilaments, but they can induce movement in the filament itself. It is this property that gives rise to the contractile properties of skeletal muscle.
Video: Sliding filament theory of muscle contraction. [Video was uploaded to YouTube by avice01 and created by Sara Egner using electron micrography from P M Motta, P M Andrews, K R Porter and J Vial.]
Contractile Bundles in Skeletal Muscle
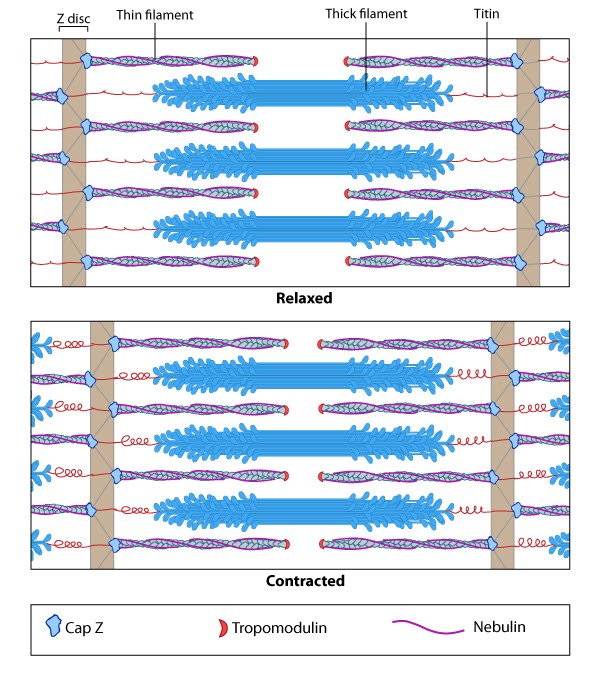
In skeletal muscle cells, myosin II forms only thick filaments that are arranged within a scaffold of actin thin filaments (along with numerous other proteins). These form the higher order fibrous structures known as sarcomeres. Each sarcomere contains numerous repeating units of interlinked thick and thin filaments, and the opposite orientation of the myosin heads causes adjacent actin filaments to slide past each other during muscle contraction. Each sarcomere is ~2 µm long in resting muscle, but this length is shortened by as much as 70% after muscle contraction. Muscle contraction is regulated by calcium levels [9] and by the troponin regulatory system. Although actin subunits continue to turn-over at both ends of the thin filament, this exchange is relatively slow, making the actin filaments in sarcomeres relatively more stable when compared to the actin filaments found in other cell types.Contractile Bundles in Nonmuscle Cells[Edit]
In non-muscle cells, myosin II associates with actin filaments to form contractile structures known as stress fibers along the lower surfaces where the cell is anchored to its substrate. In epithelial cells, contractile bundles are also prominent in the adhesion belt (aka adherens belt), which helps to maintain the stability and integrity of epithelial cell sheets. The contractile bundles in nonmuscle cells are similar to skeletal muscle fibers, but they are smaller (~0.4 µm in fibroblasts), less organized, and they contain different accessory proteins [10].
 Figure 2. Stress fiber structure: A. Isolated stress fibers have a banded appearance, with bundles of actin filaments interspersed with semiperiodic electron-dense regions. B. The electron-dense regions are rich in actin crosslinking proteins, namely α-actinin. Bipolar myosin II filaments lie between the loosely packed actin filaments in the regions that lack α-actinin . C. A high resolution view of the bipolar myosin filament heads interspersed between the regions rich in α-actinin. Relative to α-actinin, the more flexible actin crosslinking protein, filamin, is dispersed throughout the entire stress fiber.
Figure 2. Stress fiber structure: A. Isolated stress fibers have a banded appearance, with bundles of actin filaments interspersed with semiperiodic electron-dense regions. B. The electron-dense regions are rich in actin crosslinking proteins, namely α-actinin. Bipolar myosin II filaments lie between the loosely packed actin filaments in the regions that lack α-actinin . C. A high resolution view of the bipolar myosin filament heads interspersed between the regions rich in α-actinin. Relative to α-actinin, the more flexible actin crosslinking protein, filamin, is dispersed throughout the entire stress fiber.
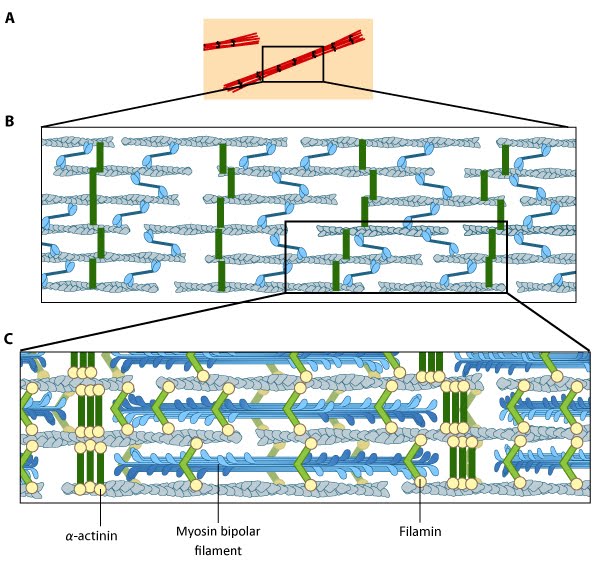 Figure 2. Stress fiber structure: A. Isolated stress fibers have a banded appearance, with bundles of actin filaments interspersed with semiperiodic electron-dense regions. B. The electron-dense regions are rich in actin crosslinking proteins, namely α-actinin. Bipolar myosin II filaments lie between the loosely packed actin filaments in the regions that lack α-actinin . C. A high resolution view of the bipolar myosin filament heads interspersed between the regions rich in α-actinin. Relative to α-actinin, the more flexible actin crosslinking protein, filamin, is dispersed throughout the entire stress fiber.
Figure 2. Stress fiber structure: A. Isolated stress fibers have a banded appearance, with bundles of actin filaments interspersed with semiperiodic electron-dense regions. B. The electron-dense regions are rich in actin crosslinking proteins, namely α-actinin. Bipolar myosin II filaments lie between the loosely packed actin filaments in the regions that lack α-actinin . C. A high resolution view of the bipolar myosin filament heads interspersed between the regions rich in α-actinin. Relative to α-actinin, the more flexible actin crosslinking protein, filamin, is dispersed throughout the entire stress fiber.Historically speaking, the mechanism of actomyosin contraction for nonmuscle actin was examined using amoebae proteins Dictyostelium, Acanthamoeba) because the actin is very similar to muscle actin [11]; these initial studies showed the rate of ATP hydrolysis by myosin (and hence myosin movement) varies directly with the actin concentration [12]. Further studies using isolated stress fibers from fibroblasts confirmed that stress fibers are contractile and shorten by as much as 25% [13]. Myosin II bundle formation and contractile activity in nonmuscle cells is regulated by phosphorylation [14].