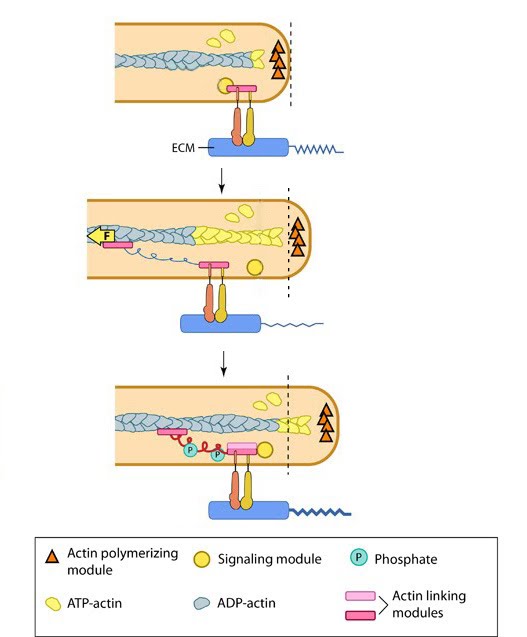
Steps in Formation 1. Initiation and Nucleation 2. Extension, Pause and Stasis 3. Formation of Adhesions 4. Force Generation and Translocation 5. Disassembly of actin filaments and retraction of the trailing edge Functional Modules
| Lamellipodia and Lamella
As the lamellipodia continues to spread and expand forward, adhesion sites form along the leading edge (see video below). These sites not only provide traction for the cells forward movement, but permit the cell to sense and measure the rigidity of its substrate. Formation of these adhesions is a complex process, involving a number of steps and functional modules. |
References
- Galbraith CG., Yamada KM. & Galbraith JA. Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science 2007; 315(5814):992-5. [PMID: 17303755]
- Giannone G., Mège RM. & Thoumine O. Multi-level molecular clutches in motile cell processes. Trends Cell Biol. 2009; 19(9):475-86. [PMID: 19716305]
- Parsons JT., Horwitz AR. & Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010; 11(9):633-43. [PMID: 20729930]
- Gardel ML., Schneider IC., Aratyn-Schaus Y. & Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu. Rev. Cell Dev. Biol. 2010; 26:315-33. [PMID: 19575647]
- Alexandrova AY., Arnold K., Schaub S., Vasiliev JM., Meister JJ., Bershadsky AD. & Verkhovsky AB. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE 2008; 3(9):e3234. [PMID: 18800171]
- Suter DM. & Forscher P. Substrate-cytoskeletal coupling as a mechanism for the regulation of growth cone motility and guidance. J. Neurobiol. 2000; 44(2):97-113. [PMID: 10934315]
- Jurado C., Haserick JR. & Lee J. Slipping or gripping? Fluorescent speckle microscopy in fish keratocytes reveals two different mechanisms for generating a retrograde flow of actin. Mol. Biol. Cell 2005; 16(2):507-18. [PMID: 15548591]
- Guo WH. & Wang YL. Retrograde fluxes of focal adhesion proteins in response to cell migration and mechanical signals. Mol. Biol. Cell 2007; 18(11):4519-27. [PMID: 17804814]
- Jiang G., Giannone G., Critchley DR., Fukumoto E. & Sheetz MP. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 2003; 424(6946):334-7. [PMID: 12867986]
- Brown CM., Hebert B., Kolin DL., Zareno J., Whitmore L., Horwitz AR. & Wiseman PW. Probing the integrin-actin linkage using high-resolution protein velocity mapping. J. Cell. Sci. 2006; 119(Pt 24):5204-14. [PMID: 17158922]
- Hu K., Ji L., Applegate KT., Danuser G. & Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science 2007; 315(5808):111-5. [PMID: 17204653]
- Assoian RK. & Klein EA. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 2008; 18(7):347-52. [PMID: 18514521]
- Reddig PJ. & Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005; 24(3):425-39. [PMID: 16258730]