Contents
1.1 Role of Mechanobiology in Shaping Cells and Tissues 1.2 Common Themes In Mechanobiology 1.3 Types of Mechanosensing 1.4 Types of Forces Cells Encounter 1.5 The Dynamic Cytoskeleton 1.6 Common Features of Polymeric Cytoskeletal Systems 1.7 How Does the Cytoskeleton Transmit Mechanical Forces? | Essential Info: What is Mechanobiology?1.6 Common Features of Polymeric Cytoskeletal SystemsAmong the three cytoskeletal components (microtubules, actin filaments and intermediate filaments), the assembly and organization of actin filaments and microtubules share more common features with each other than they do with intermediate filaments (IFs). These features, as reviewed in [1] and [2], are described below:1) Filament systems are built from smaller subunits Although the cytoskeleton can extend the entire length of a eukaryotic cell (on average 10-100 μm), the individual components are quite small (4-8 nm average) and readily diffuse in the cytoplasm. End-to-end assembly of smaller subunits forms protofilaments, followed by the lateral assembly of the protofilaments into larger polymeric filaments. These are more stable due to increased lateral interactions between the monomeric filaments and the assembly process is from the ends. The soluble subunits of the three filament systems are:
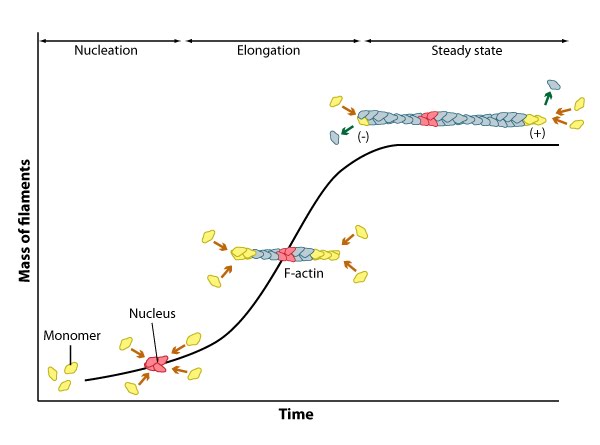
2) Filament assembly is energized by nucleotide hydrolysis Cytoskeleton protein subunits bind to nucleotide triphosphates (ATP or GTP) before joining a filament [3] with the exception of intermediate filaments [4]. The nucleotide is hydrolyzed once the subunit is incorporated into the filament. Although this weakens the non-covalent interactions of the subunits and causes structural changes to the filament, much of the released free energy is stored in the polymer lattice. The rate of nucleotide hydrolysis controls filament stability and dynamics. Differences in the orientation of filaments, numbers of filaments, presence of cross-linking proteins and the number of longitudinal and lateral interactions between filaments also regulate the stability and flexibility of the polymer. 3) Filament assembly proceeds in three phases All three filament systems self-associate to form helical structures [5, 6, 7], with intermediate filaments comprising helical dimers [5] and the final compositions of both microtubules [8] and actin filaments [9] forming left- and right-handed helices respectively. Self-association is driven by non-covalent interactions between protein subunits. These interactions are relatively weak when compared to covalent linkages, allowing these structures to rapidly assemble and disassemble, particularly in the case of microtubules and actin filaments. i) Nucleation phase: Formation of stable multimers of soluble subunits is called nucleation. Actin filaments require a seed of three or more monomers, whereas microtubules require 16 or more dimers to proceed to elongation. Nucleation complexes are activated at specific loci in cells to control the timing and polarity of filament formation. The time spent nucleating filaments varies depending on the monomer concentration. Accessory proteins can influence the site of nucleation, rate of nucleation (by altering free monomer concentration) and can stabilize the filaments to maintain a constant structure over time. ii) Elongation phase: Once a stable seed is formed subunits assemble rapidly onto the ends of the nucleated filament. E.g. for intermediate filaments, the annealing of two unit-length filaments (ULFs), from which subsequent ULFs can be added [10]. iii) Steady state phase: As the filament number and length increases, equilibrium is reached whereby the rate of subunit assembly and disassembly are evenly balanced. This equilibrium point is termed the critical concentration or Cc and it also represents the concentration of free subunits left in solution.
Figure: Three phases of filament assembly.
4) Rate of filament assembly at the two ends are different For microtubules and actin filaments, the two ends of the filament are structurally different and have dramatically different rates of subunit assembly/disassembly. This gives directionality to filament growth, with one dynamic end that grows and shrinks rapidly (plus end) and one end that grows and shrinks slowly (minus end) [11, 12]. For filament assembly, subunits of microtubules and actin hydrolyze GTP and ATP respectively. The fast growing end requires high energy bonds and as such subunits at this end preferentially bind GTP or ATP subunits, while the slower growing end will likely have a GDP or ADP bound subunit aiding disassembly [3]. Intermediate filaments do not bind nucleotides and so do not show directionality during assembly. This means they are able to gain and lose subunits along their entire length, without showing a preference to a particular end [4]. |
References
- Mitchison TJ. Compare and contrast actin filaments and microtubules. Mol. Biol. Cell 1992; 3(12):1309-15. [PMID: 1493331]
- Herrmann H., Strelkov SV., Burkhard P. & Aebi U. Intermediate filaments: primary determinants of cell architecture and plasticity. J. Clin. Invest. 2009; 119(7):1772-83. [PMID: 19587452]
- Carlier MF. Guanosine-5′-triphosphate hydrolysis and tubulin polymerization. Review article. Mol. Cell. Biochem. 1982; 47(2):97-113. [PMID: 6755216]
- Colakoğlu G. & Brown A. Intermediate filaments exchange subunits along their length and elongate by end-to-end annealing. J. Cell Biol. 2009; 185(5):769-77. [PMID: 19468066]
- Smith TA., Strelkov SV., Burkhard P., Aebi U. & Parry DA. Sequence comparisons of intermediate filament chains: evidence of a unique functional/structural role for coiled-coil segment 1A and linker L1. J. Struct. Biol.; 137(1-2):128-45. [PMID: 12064940]
- Sept D., Baker NA. & McCammon JA. The physical basis of microtubule structure and stability. Protein Sci. 2003; 12(10):2257-61. [PMID: 14500883]
- Fujii T., Iwane AH., Yanagida T. & Namba K. Direct visualization of secondary structures of F-actin by electron cryomicroscopy. Nature 2010; 467(7316):724-8. [PMID: 20844487]
- Mandelkow EM., Schultheiss R., Rapp R., Müller M. & Mandelkow E. On the surface lattice of microtubules: helix starts, protofilament number, seam, and handedness. J. Cell Biol. 1986; 102(3):1067-73. [PMID: 3949873]
- Holmes KC., Popp D., Gebhard W. & Kabsch W. Atomic model of the actin filament. Nature 1990; 347(6288):44-9. [PMID: 2395461]
- Kirmse R., Portet S., Mücke N., Aebi U., Herrmann H. & Langowski J. A quantitative kinetic model for the in vitro assembly of intermediate filaments from tetrameric vimentin. J. Biol. Chem. 2007; 282(25):18563-72. [PMID: 17403663]
- Pollard TD. Assembly and dynamics of the actin filament system in nonmuscle cells. J. Cell. Biochem. 1986; 31(2):87-95. [PMID: 3525579]
- Summers K. & Kirschner MW. Characteristics of the polar assembly and disassembly of microtubules observed in vitro by darkfield light microscopy. J. Cell Biol. 1979; 83(1):205-17. [PMID: 511939]