Contents
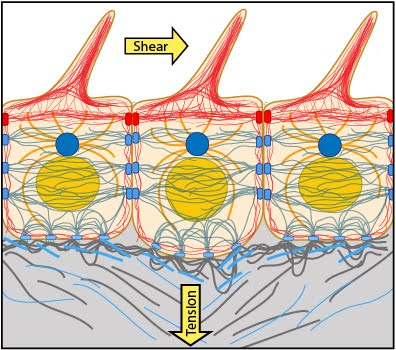
1.1 Role of Mechanobiology in Shaping Cells and Tissues 1.2 Common Themes In Mechanobiology 1.3 Types of Mechanosensing 1.4 Types of Forces Cells Encounter 1.5 The Dynamic Cytoskeleton 1.6 Common Features of Polymeric Cytoskeletal Systems 1.7 How Does the Cytoskeleton Transmit Mechanical Forces? | Essential Info: What is Mechanobiology?1.4 Types of forces cells may encounterCells and subcellular structures experience forces from a variety of sources. In general, forces are developed from within the cell via the cytoskeleton (endogenous forces) or come from outside the cell (applied forces). Forces exerted on the cell are often dynamic in nature, requiring the cell to constantly re-evaluate its status and adjust its internal and external morphology accordingly. Although the mechanosensors and mechanotransduction events occur locally at the cell periphery, the forces and biochemical signals are transmitted throughout the cell [1, 2, 3, 4] and are integrated over time [5, 6]. In general, they promote stiffening, softening and reorientation of cytoskeletal filaments [7, 8, 9]. For more details, see Chapter: Actin filaments are a force-sensing conduit. This results in strengthening of the entire contractile machinery without disturbing cellular connectivity (reviewed in [10]).Endogenous forcesDuring cytoskeletal assembly, the filament subunits encounter intermolecular bonding forces that attract (pulls or tenses) its neighboring subunits. These forces are counterbalanced by intramolecular resistance against being compressed. The stiff and flexible regions present in most components and associated proteins endow filaments with elasticity and ‘pre-stresses’ the entire system to resist deforming forces such as extension, bending and compression (reviewed in [11, 12]).In non-muscle cells, contractile resistance to deformation (upon force/ stress application) is provided by the actoyosin machinery. This machinery generates tensional forces by remodeling and exerts traction forces on cell-cell and cell-matrix adhesions (strain), thereby creating a resting tension within the cell (reviewed in [12, 13]) (See ‘Figure: Contractile machinery provides resistance to deformation’ below). This contractile tension is representative of the feedback system that a cell uses to couple external and internal mechanotransduction events (reviewed in [14]). Variation in the stress vs. strain homeostasis can influence which of the different mechanosensors work together to orchestrate a concerted response and governs how the signal is integrated [15, 16]. Figure: Contractile machinery provides resistance to deformation. Each of the three filament types that constitute the internal cytoskeleton, e.g., actin filaments (shown in red), microtubules (shown in yellow), and intermediate filaments (shown in blue-gray), are interlinked in a state of resting tension throughout the cell, to other cells, and to the ECM. Applied forcesExternal forces experienced by cells and tissues include tension, compression, shear, swelling and membrane curvature. Resilient and pre-stressed, the contractile machinery immediately responds to micrometric and nanometric variations in the geometry, topography or spatial distribution of their environment [17, 18, 19, 20, 21, 22] (See ‘Figure: Deforming forces initiate mechanotransduction events’ below).Forces applied at the macroscale cause a change in the strength of cell-cell or cell-matrix associations. This activates mechanosensors and signals down through the cellular network via the cell membrane, adhesion receptors and focal adhesions. Specific pathways focus the force onto protein complexes that comprise functional modules [23, 24, 25]. These mechanotransduction events allow the cell to distinguish the chemical nature [26, 27] and stiffness of the underlying surface [28], as well as specific types of extracellular matrix (ECM) fibers [29]. These factors influence the downstream signaling and cytoskeletal events that lead to altered cell morphology [30]. Strengthening of the contractile machinery in response to an external mechanical force, ultimately creates an opposite force within the ECM. This leads to ECM remodeling to regain homeostasis and reinforcement of adhesions to resist higher forces [1]. This process therefore creates a feedback loop within the mechano-sensing and -transduction system. Certain components of the cytoskeleton, namely the microtubules and actin filaments, bear compressive forces to counterbalance the tensile forces in the pre-stressed elements. Other elements, namely intermediate filaments, are necessary for long force transfer through the cytoplasm and for structural integration of the cytoplasm and nucleus (reviewed in [12, 10, 31]). Figure: Deforming forces initiate mechanotransduction events. Applied forces (such as shear stress or tension) are detected by mechanosensors located near the cell surface where the stress is applied. The resulting force-induced conformational changes on the mechanosensory molecules is transmitted throughout the interlinked cytoskeletal system and is focused onto other mechanosensors within the network. A concerted global mechanoresponse to the applied force(s) can influence gene expression not only through direct signal transduction pathways, but also through force transmission through the cytoskeletal elements and interlinked nuclear membrane. |
References
- Choquet D., Felsenfeld DP. & Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 1997; 88(1):39-48. [PMID: 9019403]
- Riveline D., Zamir E., Balaban NQ., Schwarz US., Ishizaki T., Narumiya S., Kam Z., Geiger B. & Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 2001; 153(6):1175-86. [PMID: 11402062]
- Galbraith CG., Yamada KM. & Sheetz MP. The relationship between force and focal complex development. J. Cell Biol. 2002; 159(4):695-705. [PMID: 12446745]
- von Wichert G., Jiang G., Kostic A., De Vos K., Sap J. & Sheetz MP. RPTP-alpha acts as a transducer of mechanical force on alphav/beta3-integrin-cytoskeleton linkages. J. Cell Biol. 2003; 161(1):143-53. [PMID: 12682088]
- Discher DE., Janmey P. & Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 2005; 310(5751):1139-43. [PMID: 16293750]
- Giannone G. & Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006; 16(4):213-23. [PMID: 16529933]
- Gardel ML., Nakamura F., Hartwig JH., Crocker JC., Stossel TP. & Weitz DA. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc. Natl. Acad. Sci. U.S.A. 2006; 103(6):1762-7. [PMID: 16446458]
- Chaudhuri O., Parekh SH. & Fletcher DA. Reversible stress softening of actin networks. Nature 2007; 445(7125):295-8. [PMID: 17230186]
- Katsumi A., Milanini J., Kiosses WB., del Pozo MA., Kaunas R., Chien S., Hahn KM. & Schwartz MA. Effects of cell tension on the small GTPase Rac. J. Cell Biol. 2002; 158(1):153-64. [PMID: 12105187]
- Ingber DE. Mechanical control of tissue growth: function follows form. Proc. Natl. Acad. Sci. U.S.A. 2005; 102(33):11571-2. [PMID: 16091458]
- Zanotti G. & Guerra C. Is tensegrity a unifying concept of protein folds? FEBS Lett. 2003; 534(1-3):7-10. [PMID: 12527354]
- Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog. Biophys. Mol. Biol.; 97(2-3):163-79. [PMID: 18406455]
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997; 59:575-99. [PMID: 9074778]
- Chen CS. Mechanotransduction – a field pulling together? J. Cell. Sci. 2008; 121(Pt 20):3285-92. [PMID: 18843115]
- Chen CS., Alonso JL., Ostuni E., Whitesides GM. & Ingber DE. Cell shape provides global control of focal adhesion assembly. Biochem. Biophys. Res. Commun. 2003; 307(2):355-61. [PMID: 12859964]
- Polte TR., Eichler GS., Wang N. & Ingber DE. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am. J. Physiol., Cell Physiol. 2004; 286(3):C518-28. [PMID: 14761883]
- Chen CS., Mrksich M., Huang S., Whitesides GM. & Ingber DE. Geometric control of cell life and death. Science 1997; 276(5317):1425-8. [PMID: 9162012]
- Curtis A. & Wilkinson C. New depths in cell behaviour: reactions of cells to nanotopography. Biochem. Soc. Symp. 1999; 65:15-26. [PMID: 10320930]
- Cukierman E., Pankov R., Stevens DR. & Yamada KM. Taking cell-matrix adhesions to the third dimension. Science 2001; 294(5547):1708-12. [PMID: 11721053]
- Geiger B. & Bershadsky A. Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell 2002; 110(2):139-42. [PMID: 12150922]
- Bershadsky AD., Balaban NQ. & Geiger B. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003; 19:677-95. [PMID: 14570586]
- Rumpler M., Woesz A., Dunlop JW., van Dongen JT. & Fratzl P. The effect of geometry on three-dimensional tissue growth. J R Soc Interface 2008; 5(27):1173-80. [PMID: 18348957]
- Wang N., Butler JP. & Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science 1993; 260(5111):1124-7. [PMID: 7684161]
- Gillespie PG. & Walker RG. Molecular basis of mechanosensory transduction. Nature 2001; 413(6852):194-202. [PMID: 11557988]
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006; 20(7):811-27. [PMID: 16675838]
- Hersel U., Dahmen C. & Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003; 24(24):4385-415. [PMID: 12922151]
- Silva GA., Czeisler C., Niece KL., Beniash E., Harrington DA., Kessler JA. & Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 2004; 303(5662):1352-5. [PMID: 14739465]
- Engler AJ., Sen S., Sweeney HL. & Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126(4):677-89. [PMID: 16923388]
- Zaidel-Bar R., Cohen M., Addadi L. & Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem. Soc. Trans. 2004; 32(Pt3):416-20. [PMID: 15157150]
- Corbett SA. & Schwarzbauer JE. Modulation of protein tyrosine phosphorylation by the extracellular matrix. J. Surg. Res. 1997; 69(1):220-5. [PMID: 9202674]
- Wang N. & Suo Z. Long-distance propagation of forces in a cell. Biochem. Biophys. Res. Commun. 2005; 328(4):1133-8. [PMID: 15707995]