Integrin Activation
Content
Introduction to Integrin Activation[Edit]
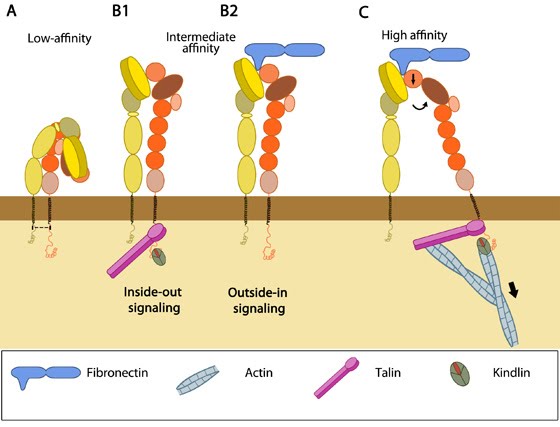
Integrin activation is an important mechanism through which cells regulate integrin function by manipulating the ligand affinity of integrins spatially and temporally. Structural and functional studies suggest that integrins can exist in different ligand affinity states – low, intermediate and high (reviewed in [1]). Crystal structures have revealed that integrin heterodimers, occur in an inactive, bent V-shape with the head close to the membrane-proximal regions of the legs [2, 3], maintained by the α/β salt bridge at the inner membrane region and helix packing of the transmembrane (TM) region [4]. This low affinity structure undergoes rapid, reversible conformational changes to increase ligand affinity, termed “activation” (reviewed in [5, 6, 7]).
The Structural hallmarks of integrin activation are:
a) complete extension of the extracellular domains and
b) separation of the cytoplasmic leg domains (structural rearrangements detailed in [8, 9]).
This process facilitates integrin-mediated signaling, thus mechano-sensing and -transmitting.
Integrin can be activated from two directions, from the inside by the regulated binding of proteins to the cytoplasmic tails, and from the outside by multivalent ligand binding. In either case, talin binding to the integrin β tails is an essential and the final common step ([10], reviewed in [11]). Though the two processes are conceptually separate, they are mutually cooperative i.e one can lead to the other. Some structural studies done with force application to mimic ligand/intracellular protein suggested that the combined action of these two events favor the transition from the closed, low affinity to a open, high affinity conformation of integrin [12]. Activation leads to bidirectional signaling crucial in a variety of anchorage-dependent events such as adhesion, cell spreading, migration, polarity and organization of the ECM leading to physiological changes (reviewed in [13]).
Here we discuss only well-established events that occur at the close proximity of integrin molecules localized on the plasma membrane.
a) complete extension of the extracellular domains and
b) separation of the cytoplasmic leg domains (structural rearrangements detailed in [8, 9]).
This process facilitates integrin-mediated signaling, thus mechano-sensing and -transmitting.
Integrin can be activated from two directions, from the inside by the regulated binding of proteins to the cytoplasmic tails, and from the outside by multivalent ligand binding. In either case, talin binding to the integrin β tails is an essential and the final common step ([10], reviewed in [11]). Though the two processes are conceptually separate, they are mutually cooperative i.e one can lead to the other. Some structural studies done with force application to mimic ligand/intracellular protein suggested that the combined action of these two events favor the transition from the closed, low affinity to a open, high affinity conformation of integrin [12]. Activation leads to bidirectional signaling crucial in a variety of anchorage-dependent events such as adhesion, cell spreading, migration, polarity and organization of the ECM leading to physiological changes (reviewed in [13]).
Here we discuss only well-established events that occur at the close proximity of integrin molecules localized on the plasma membrane.
2_Integrin Activation[Edit]
Structural hallmarks of integrin activation are:
a) complete extension of the extracellular domains and
b) separation of the cytoplasmic leg domains (structural rearrangements detailed in [8, 9]).
This process facilitates integrin-mediated signaling, thus mechano-sensing and -transmitting.
Integrin can be activated from two directions, from the inside by the regulated binding of proteins to the cytoplasmic tails, and from the outside by multivalent ligand binding. In either case, talin binding to the integrin β tails is an essential and the final common step ([10], reviewed in [11]). Though the two processes are conceptually separate, they are mutually cooperative i.e one can lead to the other. Some structural studies done with force application to mimic ligand/intracellular protein suggested that the combined action of these two events favor the transition from the closed, low affinity to a open, high affinity conformation of integrin [12]. Activation leads to bidirectional signaling crucial in a variety of anchorage-dependent events such as adhesion, cell spreading, migration, polarity and organization of the ECM leading to physiological changes (reviewed in [13]).
Here we discuss only well-established events that occur at the close proximity of integrin molecules localized on the plasma membrane. Different signaling pathways can initiate integrin activation via:
a) complete extension of the extracellular domains and
b) separation of the cytoplasmic leg domains (structural rearrangements detailed in [8, 9]).
This process facilitates integrin-mediated signaling, thus mechano-sensing and -transmitting.
Integrin can be activated from two directions, from the inside by the regulated binding of proteins to the cytoplasmic tails, and from the outside by multivalent ligand binding. In either case, talin binding to the integrin β tails is an essential and the final common step ([10], reviewed in [11]). Though the two processes are conceptually separate, they are mutually cooperative i.e one can lead to the other. Some structural studies done with force application to mimic ligand/intracellular protein suggested that the combined action of these two events favor the transition from the closed, low affinity to a open, high affinity conformation of integrin [12]. Activation leads to bidirectional signaling crucial in a variety of anchorage-dependent events such as adhesion, cell spreading, migration, polarity and organization of the ECM leading to physiological changes (reviewed in [13]).
Here we discuss only well-established events that occur at the close proximity of integrin molecules localized on the plasma membrane. Different signaling pathways can initiate integrin activation via:
Inside-out signaling versus Outside-in signaling[Edit]
Different signaling pathways can initiate integrin activation via:
a) Inside-out signaling
Signals received by other receptors foster the binding of talin and kindlin to cytoplasmic end of the integrin β subunit [14], at sites of actin polymerization. Substantial information on signaling pathway leading activation is available for integrin αIIbβ3 [15].Talin binds to integrin β-tail via F3 phospho-tyrosine binding (PTB) domain [16], a unique interaction with the membrane proximal (MP) region of the integrin (NPxY motif). This permits competition between conserved lysine on talin and an aspartic acid on integrin α essential for α/β salt bridge disruption and sufficient for integrin activation [17, 18]. Addition interactions through the basic patches in the FERM subdomain F2 helps to orient the β-subunit to promote spatial separation of the cytoplasmic domains [19, 20].
Kindlin is also an essential co-activator of integrin [21, 22] and binds to a membrane distal NxxY motif on β-integrin via its FERM F3 subdomain [23]. A preceding threonine patch on integrins β1 and β3 that gets phosphorylated [23, 24] and a tryptophan on kindlin F3 are also required for binding. However, kindlins are not known to activate integrins on their own but may render integrin-specific effects (reviewed in [25]).
The mechanism of crosstalk between integrin, talin and kindlin are not well established (reviewed in [26]). However, substantial data on the order of their binding is available. Latest Findings Talin is recruited directly to FAs from the cytosol suggesting that it does not bind to free diffusing integrins outside FAs [27] and also requires vinculin and F-actin for its activation [28]. Hence it is believed that only F-actin anchored talin at FAs bind free diffusing integrin promoting its activation [27]. Talin can directly connect to actin while kindlin links through adaptors such as migfilin, filamin, FAK, VASP and α-actinin (reviewed in [25]).
b) Outside-in signaling
Ligand binding to external domain causes conformational changes that increase ligand affinity, modify protein-interaction sites in the cytoplasmic domains and thence the resulting signals.
Besides conformational changes that extend integrin dimers ([29], reviewed in [13, 30]), multivalent ligand binding leads to clustering of integrins, which in turn activates Src family of kinases (SFKs) by autophosphorylation [31]. SFKs phosphorylate tyrosines of the integrin cytoplasmic domain (NPxY motifs) [32, 33] and other proteins [34, 35] leading to
a) control of ligand binding strength
b) alteration of binding with signaling molecules (kinases, GTPases and adaptors) [36], that constitute dynamic adhesion structures such as focal adhesions and podosomes (reviewed in [37, 38]).
Besides conformational changes that extend integrin dimers ([29], reviewed in [13, 30]), multivalent ligand binding leads to clustering of integrins, which in turn activates Src family of kinases (SFKs) by autophosphorylation [31]. SFKs phosphorylate tyrosines of the integrin cytoplasmic domain (NPxY motifs) [32, 33] and other proteins [34, 35] leading to
a) control of ligand binding strength
b) alteration of binding with signaling molecules (kinases, GTPases and adaptors) [36], that constitute dynamic adhesion structures such as focal adhesions and podosomes (reviewed in [37, 38]).
Integrin extension[Edit]
Upon alteration in the transmembrane and their proximal domains, the bent headpiece extends in less than 1 second [5] with intermediate affinity for ligands. Two models- “switchblade” and “deadbolt”- have been proposed for the mechanism of transmission of signals from across the plasma membrane leading to extension (reviewed in [1, 30]).
According to the former model, leg separation causes a jackknife-like extension of the knee that releases the hybrid domain from the constraint of the bent form [8, 39]. The latter model postulates that the activated integrin remains in a bent state (even with bound ligand) until the interaction between the headpiece and the β-stalk is disrupted by piston-like movement of TM domains and sliding of the extracellular stalks [40].
Subsequent opening of the βA/hybrid domain hinge happens by spontaneous swing out of the hybrid domain and thus the integrin dimer becomes competent for ligand binding [9]. In immunologically relevant integrins, serine phosphorylation of integrin α subunit has also been shown as a critical criterion for αI conformation changes for ligand binding [41, 42].
Tension generated by the interplay of cytoskeletal forces and ECM stiffness has been shown to be sufficient for mechanical activation of integrins to aid cell motility [43]. Nevertheless, they are believed to constantly switch between ligand bound active and unbound inactive states, thus conferring the focal adhesions with distinct dynamics so as to endure rapid changes in force [44]. Also, bent integrins that move along the cell membrane may collide with other membrane proteins and this could result in structural changes [12]. However, further structural studies in physiologically relevant conditions are required to substantially establish this theory.
According to the former model, leg separation causes a jackknife-like extension of the knee that releases the hybrid domain from the constraint of the bent form [8, 39]. The latter model postulates that the activated integrin remains in a bent state (even with bound ligand) until the interaction between the headpiece and the β-stalk is disrupted by piston-like movement of TM domains and sliding of the extracellular stalks [40].
Subsequent opening of the βA/hybrid domain hinge happens by spontaneous swing out of the hybrid domain and thus the integrin dimer becomes competent for ligand binding [9]. In immunologically relevant integrins, serine phosphorylation of integrin α subunit has also been shown as a critical criterion for αI conformation changes for ligand binding [41, 42].
Tension generated by the interplay of cytoskeletal forces and ECM stiffness has been shown to be sufficient for mechanical activation of integrins to aid cell motility [43]. Nevertheless, they are believed to constantly switch between ligand bound active and unbound inactive states, thus conferring the focal adhesions with distinct dynamics so as to endure rapid changes in force [44]. Also, bent integrins that move along the cell membrane may collide with other membrane proteins and this could result in structural changes [12]. However, further structural studies in physiologically relevant conditions are required to substantially establish this theory.
Regulation of Integrin Activation[Edit]
The following classes of proteins/ events are known to enhance or inhibit integrin activation in various contexts. It must be noted that this list provides just a few examples of proteins that regulate the activation of integrin.
i. Rap1-RIAM (Talin activation) [Lafuente et al 2004, Bos 2005, Han et al 2006]
ii. WAVE [Nolz 2007]
iii. Fam38A/r-Ras (Talin activation by calpain cleavage) [McHugh 2010]
ii. PKA mediated Ser phosphorylation- α4β1 recruitment and activation at the leading edge [Han 2003, Lim 2008]
iii. Src-kinase mediated Tyr phosphorylation of beta integrin ([Anthis JBC 2009],reviewed in [Gahmberg et al 2009])- may not have downstream effect always [Meves 2011]
ii. CIB1 and its paralogues binding to α cytoplasmic tail [Yuan 2006, Denofrio 2008]
iii. PIPKIγ90 [Martel et al 2001, Ling et al 2003, Calderwood et al 2004]
1. Regulators of talin recruitment
Proteins with membrane targeting motif that bind to talin [reviewed in Shattil 2010]i. Rap1-RIAM (Talin activation) [Lafuente et al 2004, Bos 2005, Han et al 2006]
ii. WAVE [Nolz 2007]
iii. Fam38A/r-Ras (Talin activation by calpain cleavage) [McHugh 2010]
2. Phosphorylation of integrin cytoplasmic domains
i. Tyr Phosphorylation -promote reduce talin affinity, promote Dok1 binding or other PTB domain proteins [ Calderwood 2003, Anthis JBC 2009, Oxley 2008]ii. PKA mediated Ser phosphorylation- α4β1 recruitment and activation at the leading edge [Han 2003, Lim 2008]
iii. Src-kinase mediated Tyr phosphorylation of beta integrin ([Anthis JBC 2009],reviewed in [Gahmberg et al 2009])- may not have downstream effect always [Meves 2011]
3. Competitors for talin
i. Filamin and Migfilin [Kiema 2006, Lad 2008, Ithychanda 2009]ii. CIB1 and its paralogues binding to α cytoplasmic tail [Yuan 2006, Denofrio 2008]
iii. PIPKIγ90 [Martel et al 2001, Ling et al 2003, Calderwood et al 2004]