Steps in Formation 1. Initiation 2. Extension 3. Lateral movement and Stasis 4. Adherence 5. Pulling 6. Retraction and Collapse Functional Modules
| FilopodiaStep 2. Cross-linking and ExtensionProtrusion from cells occurs by a treadmilling mechanism that is common to many actin based structures [1]. According to this model, actin filaments elongate at their barbed ends at the tips of filopodia and release subunits from their pointed ends, which lie at the rear of the structure. Filopodia may assemble in a process termed “convergent elongation” from Arp2/3-nucleated lamellipodial actin filaments that have coalesced together to form parallel bundles. Under these circumstances, the barbed ends of the filaments are locally associated with each other and protected from capping [2]. This protection is provided by the activity of additional proteins, such as the Ena/VASP family of proteins, which subsequently enhance filament elongation and promote F-actin bundling thereby stimulating filopodial protrusion [2, 3, 4, 5, 6]. The bundling protein, fascin, is also enriched near the tip of the bundle and contributes to this process. Actin filaments in filopodia are unbranched [7], suggesting that assembly occurs by elongation and not by branched nucleation.Table: Filopodial Extension Rates
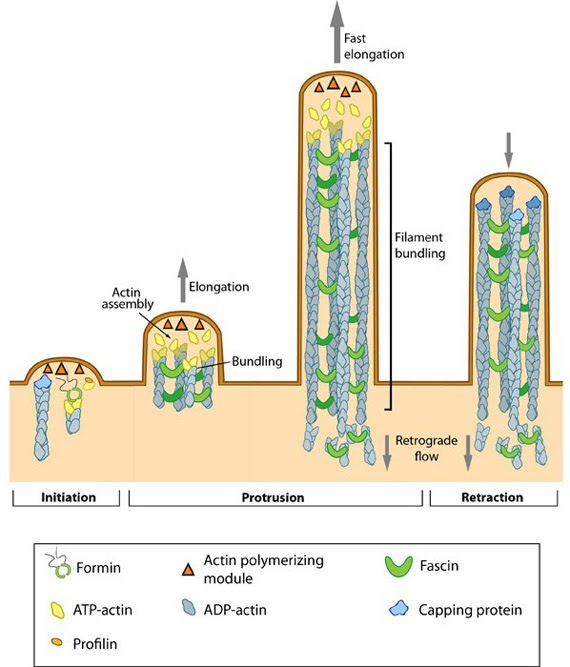
Protrusion of filopodia, produced by either convergent elongation or formin-nucleated filaments, must overcome the membrane curvature rigidity. This is aided by crosslinking actin filaments, which gives the structure the strength required to push against the compressive force of the plasma membrane [6, 15]. In nerve growth cones, more than 15 parallel filaments may be bundled together by crosslinking [16]. Bundle stiffness increases with the number of bundled filaments and so contributes to the overall length of the filopodium [6]. Many F-actin crosslinking proteins, such as α-actinin and fascin, co-localize at the base of filopodia and work in concert to produce crosslinked filaments [17]. The filamin family of proteins are also crucial actin crosslinking and scaffolding proteins and bind to both actin and a number of signaling molecules, including Rho GTPases. Crosslinking also increases the ATPase activity of myosins and increases the tension on filaments [18]. More on cross linking can be found in the Functional Module: Cross-linkers in Actin Bundling. Figure: Steps in filopodium formation. Actin filament assembly can be initiated by uncapping pre-formed actin filaments or by de novo formation (which includes both formin- and Arp2/3-mediated [not shown] nucleation). The force produced by actin assembly at the barbed end of actin filaments drives membrane protrusion. Numerous proteins (including IRSp53, Ena/VASP proteins, WASp/Scar proteins) cooperate to promote actin-assembly and enhance bundling of actin filaments by fascin. When the barbed end of the filament is capped, this stops filament assembly and protrusion; continued retrograde movement of the filaments results in retraction of the filopodium. (Figure adapted from [19]). The extension rate of a filopodium is controlled by the availability of G-actin-ATP, associated structural components and the energetics of membrane bending. The growth of long filopodia (>10 μm in length) requires the rapid transport of key materials towards the growing end [20] and this process is facilitated by the myosin X motor protein using an ATP-dependent ‘walking’ mechanism. More on this process can be found in the Functional Module: Myosin-X and Cargo Transport. |
References
- Small JV. Lamellipodia architecture: actin filament turnover and the lateral flow of actin filaments during motility. Semin. Cell Biol. 1994; 5(3):157-63. [PMID: 7919229]
- Svitkina TM., Bulanova EA., Chaga OY., Vignjevic DM., Kojima S., Vasiliev JM. & Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol. 2003; 160(3):409-21. [PMID: 12566431]
- Schirenbeck A., Arasada R., Bretschneider T., Stradal TE., Schleicher M. & Faix J. The bundling activity of vasodilator-stimulated phosphoprotein is required for filopodium formation. Proc. Natl. Acad. Sci. U.S.A. 2006; 103(20):7694-9. [PMID: 16675552]
- Lebrand C., Dent EW., Strasser GA., Lanier LM., Krause M., Svitkina TM., Borisy GG. & Gertler FB. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron 2004; 42(1):37-49. [PMID: 15066263]
- Han YH., Chung CY., Wessels D., Stephens S., Titus MA., Soll DR. & Firtel RA. Requirement of a vasodilator-stimulated phosphoprotein family member for cell adhesion, the formation of filopodia, and chemotaxis in dictyostelium. J. Biol. Chem. 2002; 277(51):49877-87. [PMID: 12388544]
- Mogilner A. & Rubinstein B. The physics of filopodial protrusion. Biophys. J. 2005; 89(2):782-95. [PMID: 15879474]
- Svitkina TM. & Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 1999; 145(5):1009-26. [PMID: 10352018]
- Sheetz MP., Wayne DB. & Pearlman AL. Extension of filopodia by motor-dependent actin assembly. Cell Motil. Cytoskeleton 1992; 22(3):160-9. [PMID: 1423662]
- Mallavarapu A. & Mitchison T. Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. J. Cell Biol. 1999; 146(5):1097-106. [PMID: 10477762]
- Schirenbeck A., Bretschneider T., Arasada R., Schleicher M. & Faix J. The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat. Cell Biol. 2005; 7(6):619-25. [PMID: 15908944]
- Tornieri K., Welshhans K., Geddis MS. & Rehder V. Control of neurite outgrowth and growth cone motility by phosphatidylinositol-3-kinase. Cell Motil. Cytoskeleton 2006; 63(4):173-92. [PMID: 16463277]
- Medalia O., Beck M., Ecke M., Weber I., Neujahr R., Baumeister W. & Gerisch G. Organization of actin networks in intact filopodia. Curr. Biol. 2007; 17(1):79-84. [PMID: 17208190]
- Costantino S., Kent CB., Godin AG., Kennedy TE., Wiseman PW. & Fournier AE. Semi-automated quantification of filopodial dynamics. J. Neurosci. Methods 2008; 171(1):165-73. [PMID: 18394712]
- Miller J., Fraser SE. & McClay D. Dynamics of thin filopodia during sea urchin gastrulation. Development 1995; 121(8):2501-11. [PMID: 7671814]
- Mogilner A. & Oster G. Cell motility driven by actin polymerization. Biophys. J. 1996; 71(6):3030-45. [PMID: 8968574]
- Lewis AK. & Bridgman PC. Nerve growth cone lamellipodia contain two populations of actin filaments that differ in organization and polarity. J. Cell Biol. 1992; 119(5):1219-43. [PMID: 1447299]
- Tseng Y., Kole TP., Lee JS., Fedorov E., Almo SC., Schafer BW. & Wirtz D. How actin crosslinking and bundling proteins cooperate to generate an enhanced cell mechanical response. Biochem. Biophys. Res. Commun. 2005; 334(1):183-92. [PMID: 15992772]
- Coleman TR. & Mooseker MS. Effects of actin filament cross-linking and filament length on actin-myosin interaction. J. Cell Biol. 1985; 101(5 Pt 1):1850-7. [PMID: 2932451]
- Faix J. & Rottner K. The making of filopodia. Curr. Opin. Cell Biol. 2006; 18(1):18-25. [PMID: 16337369]
- Schmidt CE., Dai J., Lauffenburger DA., Sheetz MP. & Horwitz AF. Integrin-cytoskeletal interactions in neuronal growth cones. J. Neurosci. 1995; 15(5 Pt 1):3400-7. [PMID: 7751919]