Cell-Matrix Adhesion
Content
The extracellular matrix and the basal lamina[Edit]
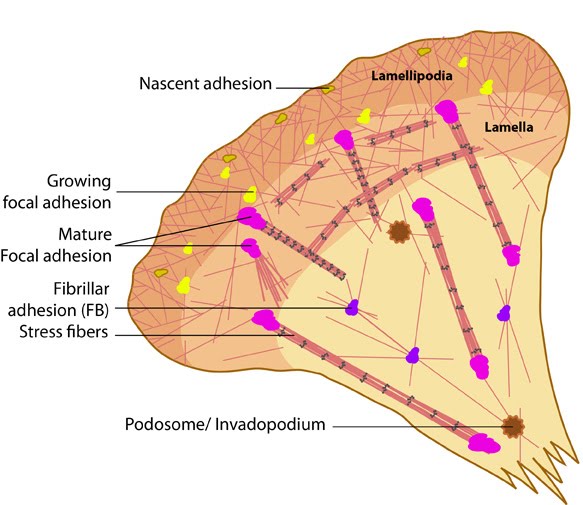
Cell-matrix adhesion is the interaction of a cell with the extracellular matrix, mediated by multi-protein adhesion structures such as focal adhesions, fibrillar adhesions and podosomes.
The ECM is a network of extracellular molecules which are secreted locally to ensure cell and tissue cohesion. The ECM also serves as a reservoir for extracellular signaling molecules that control cell growth, migration, and differentiation. The major classes of ECM molecules are proteoglycans, collagens and multi-adhesive matrix proteins (e.g. laminin, fibronectin). In mammals, the ECM is commonly known as “connective tissue”. ECM components are linked to each other through diverse protein and carbohydrate-binding domains. For stability in tissues, cells are linked to the ECM through cell adhesion receptors (e.g. integrins). A specialized form of extracellular matrix that underlies the basal side of polarized epithelial cell sheets to separate them from the underlying connective tissue is the basal lamina [1].
The ECM is a network of extracellular molecules which are secreted locally to ensure cell and tissue cohesion. The ECM also serves as a reservoir for extracellular signaling molecules that control cell growth, migration, and differentiation. The major classes of ECM molecules are proteoglycans, collagens and multi-adhesive matrix proteins (e.g. laminin, fibronectin). In mammals, the ECM is commonly known as “connective tissue”. ECM components are linked to each other through diverse protein and carbohydrate-binding domains. For stability in tissues, cells are linked to the ECM through cell adhesion receptors (e.g. integrins). A specialized form of extracellular matrix that underlies the basal side of polarized epithelial cell sheets to separate them from the underlying connective tissue is the basal lamina [1].
Basal laminae (plural) also surround individual muscle cells, fat cells, and cells lining peripheral nerve cell axons (i.e. Schwann cells) [1]. The basal lamina is thin and flexible, and is composed of closely packed matrix molecules that lack significant volume. The basal lamina components are synthesized and deposited by the cells on either side: the epithelial cells and the cells within the underlying bed of connective tissue (i.e. fibroblasts). The basal laminae forms a cohesive network and mechanical connection between cells and their external environment. Force-driven signals originating between the basal lamina components (i.e. fibronectin) and linked cell adhesion receptors (i.e. integrins) is communicated to the interior of cells through a mechanotransduction system to influence cell polarity, metabolism, fate, and migration.
The key constituents found in the basal lamina are glycoproteins (i.e. laminin, collagen) and proteoglycans (i.e. perlecan), however, the precise composition varies from tissue to tissue and various other molecules (e.g. fibronectin) can also be found [1].
The key constituents found in the basal lamina are glycoproteins (i.e. laminin, collagen) and proteoglycans (i.e. perlecan), however, the precise composition varies from tissue to tissue and various other molecules (e.g. fibronectin) can also be found [1].
What are cell-matrix adhesions?[Edit]
Cell-matrix interactions are mediated by adhesion receptors and lead to the formation of multi-protein adhesion structures that interact with the actin cytoskeleton at the cell interior; collectively, they are called cell-matrix adhesion complexes (CMACs) [2].
These adhesions act as vital information processing centers that enable cells to sense numerous extracellular signals that convey information about the chemistry, geometry, and physical properties of the ECM (reviewed in [3]). The substrate type or chemical composition (reviewed in [4]), its rigidity [5, 6], and the surface topography [7, 8, 9](reviewed in [10, 11, 12]) influence force-induced events through CMACs, and mechanosensitive cells transmit this information through subsequent mechanotransduction pathways and signaling cascades to influence diverse processes such as the cell shape, polarity, fate, motility and deposition and/or restructuring of ECM components [5, 13, 14, 15] (reviewed in [3, 11, 16, 17]).What are focal adhesions?[Edit]
Focal adhesions are the best-characterized among the different types of CMACs and are known to occur in different adhesion stages depending on the position and interplay of internal and external forces. FAs are known to evolve into fibrillar adhesions (FBs), which promote reorganization of the ECM while moving towards the center of the cell (reviewed in [18]). They are also capable of transforming into podosomes under certain conditions [19]. Such transitions could be of remarkable significance under physiological conditions.
* not all adhesions may be present within the same cell at the same time
* the relative level of adhesion types may vary (e.g. during cell motility, differentiation)
* the presence (or relative level) of a particular adhesion may be cell type- or tissue-specific Cell binding to ECM components also mediates the assembly of cell-matrix adhesions during cell migration. For example; filopodia attach to ECM components through integrin receptors, allowing these structures to probe the stiffness of the environment around them and promote migration [24]. Integrin molecules accumulate within filopodia to mediate the initial cell-matrix adhesions [25]. In addition, basal adhesions to laminin anchor the filopodial base, which usually remains immobile despite considerable flexibility in the shaft [26].
Tension that is generated between the cytoskeletal network (via the action of contractile stress fibers), linked ECM and focal adhesions controls the cells ability to migrate and protrude filopodia. Cell-matrix adhesion therefore functions as a molecular ‘clutch’ to convert intracellular cytoskeletal assembly into protrusion and movement [27]. Cells also interact with and modify ECM components mechanically, as well as chemically, to alter their alignment and composition in ways that influence cell fate, movement, polarization, and shape. E.g. through the secretion of matricellular proteins that alter ECM composition, which in turns affects cell morphology [28].
CMACs are highly dynamic and flexible, with their protein content ranging from a few components to over 100 different proteins (reviewed in [3, 20, 21]). They are assembled, disassembled, and translocated during cell spreading, polarization, migration, and division. The various types of CMACs not only differ in their basic features such as size, location, and shape, but they also differ in their composition, dynamics, component turnover and linkage to F-actin [22, 23]. It should be noted that:
* not all adhesions may be present within the same cell at the same time
* the relative level of adhesion types may vary (e.g. during cell motility, differentiation)
* the presence (or relative level) of a particular adhesion may be cell type- or tissue-specific Cell binding to ECM components also mediates the assembly of cell-matrix adhesions during cell migration. For example; filopodia attach to ECM components through integrin receptors, allowing these structures to probe the stiffness of the environment around them and promote migration [24]. Integrin molecules accumulate within filopodia to mediate the initial cell-matrix adhesions [25]. In addition, basal adhesions to laminin anchor the filopodial base, which usually remains immobile despite considerable flexibility in the shaft [26].
Tension that is generated between the cytoskeletal network (via the action of contractile stress fibers), linked ECM and focal adhesions controls the cells ability to migrate and protrude filopodia. Cell-matrix adhesion therefore functions as a molecular ‘clutch’ to convert intracellular cytoskeletal assembly into protrusion and movement [27]. Cells also interact with and modify ECM components mechanically, as well as chemically, to alter their alignment and composition in ways that influence cell fate, movement, polarization, and shape. E.g. through the secretion of matricellular proteins that alter ECM composition, which in turns affects cell morphology [28].
What are fibrillar adhesions[Edit]
Fibrillar adhesions are cell-matrix adhesion structures, that are located towards the center of a cell and are believed to evolve from mature focal adhesions. They are bound specifically to fibronectin via α5β1-integrins [29] and appear as long streaks or an array of dots [23, 18]. Fibrillar adhesions are mainly composed of thin actin cables that are crosslinked by the actin binding protein, tensin, and lack linkage to stress fibers [30, 22].
The physical state of the extracellular matrix also influences the formation of fibrillar adhesions [30] in the same way as on focal adhesions. The translocation of pliant matrix components (such as fibronectin) and increased cellular tension from the actin cables to the translocating integrins and associated fibronectin molecules is suggested to initiate fibronectin fibrillogenesis and FB assembly [29].
Fibrillar adhesions are distinguished from FAs by the high levels of tensin and low levels or absence of phosphotyrosine [30, 22, 35]. They also lack attachment to stress fibres and do not diassemble when the force is relaxed [32]. Integrin linked kinase (ILK) and the complex it forms with cytoskeleton adaptor proteins, PINCH1 and parvin, are thought to be essential for the transition from early focal adhesions to fibrillar adhesions [36]. This IPP complex functions to reinforce α5β1-actin linkage and provide a platform for tensin and zyxin recruitment.
Controversial evidences exist for the role of FAK–Src signaling pathway in regulating fibrillar adhesions and fibrillogenesis. Some studies report that loss of Src family kinases or FAK activity increases tensin recruitment [37], while in others, similar mutants show reduced efficiency in assembling fibronectin into fibrils [38, 39].
Steps in Formation
Fibrillar adhesions arise from the medial ends of growing focal adhesions at sites where α5β1-integrins translocated out of these complexes centripetally along the underlying fibronectin fibres [31]. The amount of tensin that is bound to the fibrillar adhesions increases as they are translocated [29, 32]. This directional movement towards the cell center causes stretching of the bound fibronectin dimers fibrils in the extracellular matrix (ECM) and promotes its reorganization into fibrils, driven by Rho GTPase activation of actomyosin contractility [32, 33](reviewed in [34]).The physical state of the extracellular matrix also influences the formation of fibrillar adhesions [30] in the same way as on focal adhesions. The translocation of pliant matrix components (such as fibronectin) and increased cellular tension from the actin cables to the translocating integrins and associated fibronectin molecules is suggested to initiate fibronectin fibrillogenesis and FB assembly [29].
Fibrillar adhesions are distinguished from FAs by the high levels of tensin and low levels or absence of phosphotyrosine [30, 22, 35]. They also lack attachment to stress fibres and do not diassemble when the force is relaxed [32]. Integrin linked kinase (ILK) and the complex it forms with cytoskeleton adaptor proteins, PINCH1 and parvin, are thought to be essential for the transition from early focal adhesions to fibrillar adhesions [36]. This IPP complex functions to reinforce α5β1-actin linkage and provide a platform for tensin and zyxin recruitment.
Controversial evidences exist for the role of FAK–Src signaling pathway in regulating fibrillar adhesions and fibrillogenesis. Some studies report that loss of Src family kinases or FAK activity increases tensin recruitment [37], while in others, similar mutants show reduced efficiency in assembling fibronectin into fibrils [38, 39].